Bidirectional switch of the valence associated with a hippocampal contextual memory engram
- PMID: 25162525
- PMCID: PMC4169316
- DOI: 10.1038/nature13725
Bidirectional switch of the valence associated with a hippocampal contextual memory engram
Abstract
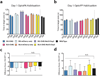
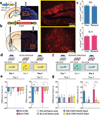
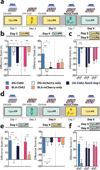
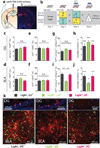
The valence of memories is malleable because of their intrinsic reconstructive property. This property of memory has been used clinically to treat maladaptive behaviours. However, the neuronal mechanisms and brain circuits that enable the switching of the valence of memories remain largely unknown. Here we investigated these mechanisms by applying the recently developed memory engram cell- manipulation technique. We labelled with channelrhodopsin-2 (ChR2) a population of cells in either the dorsal dentate gyrus (DG) of the hippocampus or the basolateral complex of the amygdala (BLA) that were specifically activated during contextual fear or reward conditioning. Both groups of fear-conditioned mice displayed aversive light-dependent responses in an optogenetic place avoidance test, whereas both DG- and BLA-labelled mice that underwent reward conditioning exhibited an appetitive response in an optogenetic place preference test. Next, in an attempt to reverse the valence of memory within a subject, mice whose DG or BLA engram had initially been labelled by contextual fear or reward conditioning were subjected to a second conditioning of the opposite valence while their original DG or BLA engram was reactivated by blue light. Subsequent optogenetic place avoidance and preference tests revealed that although the DG-engram group displayed a response indicating a switch of the memory valence, the BLA-engram group did not. This switch was also evident at the cellular level by a change in functional connectivity between DG engram-bearing cells and BLA engram-bearing cells. Thus, we found that in the DG, the neurons carrying the memory engram of a given neutral context have plasticity such that the valence of a conditioned response evoked by their reactivation can be reversed by re-associating this contextual memory engram with a new unconditioned stimulus of an opposite valence. Our present work provides new insight into the functional neural circuits underlying the malleability of emotional memory.
Figures



Comment in
-
Neuroscience: Shedding light on a change of mind.Nature. 2014 Sep 18;513(7518):323-4. doi: 10.1038/nature13745. Epub 2014 Aug 27. Nature. 2014. PMID: 25162529 No abstract available.
-
Learning and memory: uncoupling memory traces.Nat Rev Neurosci. 2014 Oct;15(10):629. doi: 10.1038/nrn3828. Epub 2014 Sep 10. Nat Rev Neurosci. 2014. PMID: 25205375 No abstract available.
Similar articles
-
Destabilization of fear memory by Rac1-driven engram-microglia communication in hippocampus.Brain Behav Immun. 2024 Jul;119:621-636. doi: 10.1016/j.bbi.2024.04.024. Epub 2024 Apr 24. Brain Behav Immun. 2024. PMID: 38670239
-
Inception of a false memory by optogenetic manipulation of a hippocampal memory engram.Philos Trans R Soc Lond B Biol Sci. 2013 Dec 2;369(1633):20130142. doi: 10.1098/rstb.2013.0142. Print 2014 Jan 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24298144 Free PMC article. Review.
-
Amygdala Reward Neurons Form and Store Fear Extinction Memory.Neuron. 2020 Mar 18;105(6):1077-1093.e7. doi: 10.1016/j.neuron.2019.12.025. Epub 2020 Jan 14. Neuron. 2020. PMID: 31952856
-
Optogenetic Activation of the Basolateral Amygdala Promotes Both Appetitive Conditioning and the Instrumental Pursuit of Reward Cues.J Neurosci. 2020 Feb 19;40(8):1732-1743. doi: 10.1523/JNEUROSCI.2196-19.2020. Epub 2020 Jan 17. J Neurosci. 2020. PMID: 31953370 Free PMC article.
-
Synaptic Plasticity, Engrams, and Network Oscillations in Amygdala Circuits for Storage and Retrieval of Emotional Memories.Neuron. 2017 May 17;94(4):731-743. doi: 10.1016/j.neuron.2017.03.022. Neuron. 2017. PMID: 28521127 Review.
Cited by
-
A circuit mechanism for differentiating positive and negative associations.Nature. 2015 Apr 30;520(7549):675-8. doi: 10.1038/nature14366. Nature. 2015. PMID: 25925480 Free PMC article.
-
An evidence-based critical review of the mind-brain identity theory.Front Psychol. 2023 Oct 27;14:1150605. doi: 10.3389/fpsyg.2023.1150605. eCollection 2023. Front Psychol. 2023. PMID: 37965649 Free PMC article.
-
A practical approach to the ethical use of memory modulating technologies.BMC Med Ethics. 2020 Sep 18;21(1):89. doi: 10.1186/s12910-020-00532-z. BMC Med Ethics. 2020. PMID: 32948166 Free PMC article.
-
Neurophotonics Approaches for the Study of Pattern Separation.Front Neural Circuits. 2020 Jun 9;14:26. doi: 10.3389/fncir.2020.00026. eCollection 2020. Front Neural Circuits. 2020. PMID: 32587504 Free PMC article. Review.
-
Granule Cell Ensembles in Mouse Dentate Gyrus Rapidly Upregulate the Plasticity-Related Protein Synaptopodin after Exploration Behavior.Cereb Cortex. 2020 Apr 14;30(4):2185-2198. doi: 10.1093/cercor/bhz231. Cereb Cortex. 2020. PMID: 31812981 Free PMC article.
References
-
- Wolpe J. Psychotherapy by reciprocal inhibition. Stanford University Press; 1958.
-
- Ramirez S, et al. Creating a false memory in the hippocampus. Science. 2013;341:387–391. - PubMed
-
- Muramoto K, Ono T, Nishijo H, Fukuda M. Rat amygdaloid neuron responses during auditory discrimination. Neuroscience. 1993;52:621–636. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

