Neonatal vitamin A supplementation associated with a cluster of deaths and poor early growth in a randomised trial among low-birth-weight boys of vitamin A versus oral polio vaccine at birth
- PMID: 25163399
- PMCID: PMC4236664
- DOI: 10.1186/1471-2431-14-214
Neonatal vitamin A supplementation associated with a cluster of deaths and poor early growth in a randomised trial among low-birth-weight boys of vitamin A versus oral polio vaccine at birth
Abstract
Background: The effect of oral polio vaccine administered already at birth (OPV0) on child survival was not examined before being recommended in 1985. Observational data suggested that OPV0 was harmful for boys, and trials have shown that neonatal vitamin A supplementation (NVAS) at birth may be beneficial for boys. We set out to test this research question in a randomised trial.
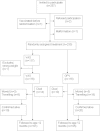
Methods: The trial was carried out at the Bandim Health Project, Guinea-Bissau. We planned to enrol 900 low-birth weight (LBW) boys in a randomised trial to investigate whether NVAS instead of OPV0 could lower infant mortality for LBW boys. At birth, the children were randomised to OPV (usual treatment) or VAS (intervention treatment) and followed for 6 months for growth and 12 months for survival. Hazard Ratios (HR) for mortality were calculated using Cox regression. We compared the individual anthropometry measurements to the 2006 WHO growth reference. We compared differences in z-scores by linear regression. Relative risks (RR) of being stunted or underweight were calculated in Poisson regression models with robust standard errors.
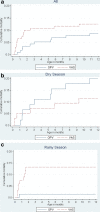
Results: In the rainy season we detected a cluster of deaths in the VAS group and the trial was halted immediately with 232 boys enrolled. The VAS group had significantly higher mortality than the OPV0 group in the rainy season (HR: 9.91 (1.23 - 80)). All deaths had had contact with the neonatal nursery; of seven VAS boys enrolled during one week in September, six died within two months of age, whereas only one died among the six boys receiving OPV (p = 0.05). Growth (weight and arm-circumference) in the VAS group was significantly worse until age 3 months.
Conclusion: VAS at birth instead of OPV was not beneficial for the LBW boys in this study. With the premature closure of the trial it was not possible to answer the research question. However, the results of this study call for extra caution when testing the effect of NVAS in the future.
Trial registration: http://www.clinicaltrials.gov NCT00625482. Registered 18 February 2008.
Figures
References
-
- West KP, Katz J, Shrestha SR, LeClerq SC, Khatry SK, Pradhan EK, Adhikari R, Wu LS, Pokhrel RP, Sommer A. Mortality of infants < 6 mo of age supplemented with vitamin A: a randomized, double-masked trial in Nepal. Am J Clin Nutr. 1995;62:143–148. - PubMed
-
- Malaba LC, Iliff PJ, Nathoo KJ, Marinda E, Moulton LH, Zijenah LS, Zvandasara P, Ward BJ, Humphrey JH. the ZVITAMBO Study Group. Effect of postpartum maternal or neonatal vitamin A supplementation on infant mortality among infants born to HIV-negative mothers in Zimbabwe. Am J Clin Nutr. 2005;81:454–460. - PubMed
-
- Humphrey JH, Iliff PJ, Marinda ET, Mutasa K, Moulton LH, Chidawanyika H, Ward BJ, Nathoo KJ, Malaba LC, Zijenah LS, Zvandasara P, Ntozini R, Mzengeza F, Mahomva AI, Ruff AJ, Mbizvo MT, Zunguza CD. Effects of a single large dose of vitamin A, given during the postpartum period to HIV-positive women and their infants, on child HIV infection, HIV-free survival, and mortality. J Infect Dis. 2006;193:860. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous