Total synthesis of myceliothermophins C, D, and E
- PMID: 25163463
- PMCID: PMC4257465
- DOI: 10.1002/anie.201406815
Total synthesis of myceliothermophins C, D, and E
Abstract
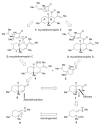
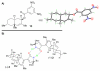
The total synthesis of cytotoxic polyketides myceliothermophins E (1), C (2), and D (3) through a cascade-based cyclization to form the trans-fused decalin system is described. The convergent synthesis delivered all three natural products through late-stage divergence and facilitated unambiguous C21 structural assignments for 2 and 3 through X-ray crystallographic analysis, which revealed an interesting dimeric structure between its enantiomeric forms.
Keywords: cascade reactions; decalin; natural products; polyketides; total synthesis.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




References
-
- Royles BJL. Chem. Rev. 1995;95:1981–2001.
- Nay B, Riache N, Evanno L. Nat. Prod. Rep. 2009;26:1044–1062. - PubMed
- Li XW, Ear A, Nay B. Nat. Prod. Rep. 2013;30:765–782. - PubMed
- Hoye TR, Dvornikovs V. J. Am. Chem. Soc. 2006;128:2550–2551. - PubMed
- Nicolaou KC, Sarlah D, Wu TR, Zhan WQ. Angew. Chem. 2009;121:7002–7006. - PMC - PubMed
- Angew. Chem. Int. Ed. 2009;48:6870–6874. - PMC - PubMed
- Nicolaou KC, Sun Y-P, Sarlah D, Zhan WQ, Wu TR. Org. Lett. 2011;13:5708–5710. - PMC - PubMed
- Deng J, Zhu B, Lu Z, Yu H, Li A. J. Am. Chem. Soc. 2012;134:920–923. - PubMed
- Uchida K, Ogawa T, Yasuda Y, Mimura H, Fujimoto T, Fukuyama T, Wakimoto T, Asakawa T, Hamashima Y, Kan T. Angew. Chem. 2012;124:13022–13025. - PubMed
- Angew. Chem. Int. Ed. 2012;51:12850–12853. - PubMed
- Yin J, Kong L, Wang C, Shi Y, Cai S, Gao S. Chem. Eur. J. 2013;19:13040–13046. - PubMed
-
- Yang YL, Lu CP, Chen MY, Chen KY, Wu YC, Wu SH. Chem. Eur. J. 2007;13:6985–6991. - PubMed
-
-
Shionozaki N, Yamaguchi T, Kitano H, Tomizawa M, Makino K, Uchiro H. Tetrahedron Lett. 2012;53:5167–5170. (Note: No detailed experimental procedures or physical data of intermediates were provided in this publication). For natural products containing similar trans-fused decalin systems, see: Singh SB, Goetz MA, Jones ET, Bills GF, Giacobbe RA, Herranz L, Miles SS, Williams DL. J. Org. Chem. 1995;60:7040–7042. West RR, Van Ness J, Varming A-M, Rassing B, Biggs S, Gasper S, Mckernan PA, Piggot J. J. Antibiot. 1996;49:967–973. Suzuki S, Hosoe T, Nozawa K, Kawai K, Yaguchi T, Udagawa S. J. Nat. Prod. 2000;63:768–772. Pornpakakul S, Roengsumran S, Deechangvipart S, Petsom A, Muangsin N, Ngamrojnavanich N, Sriubolmas N, Chaichit N, Ohta T. Tetrahedron Lett. 2007;48:651–655. Kontnik R, Clardy J. Org. Lett. 2008;10:4149–4151.
-
-
- Watanabe H, Onoda T, Kitahara T. Tetrahedron Lett. 1999;40:2545–2548.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

