Influence of insulin-like growth factor I overexpression via recombinant adeno-associated vector gene transfer upon the biological activities and differentiation potential of human bone marrow-derived mesenchymal stem cells
- PMID: 25163769
- PMCID: PMC4164762
- DOI: 10.1186/scrt491
Influence of insulin-like growth factor I overexpression via recombinant adeno-associated vector gene transfer upon the biological activities and differentiation potential of human bone marrow-derived mesenchymal stem cells
Abstract
Introduction: The transplantation of genetically modified progenitor cells such as bone marrow-derived mesenchymal stem cells (MSCs) is an attractive strategy to improve the natural healing of articular cartilage defects. In the present study, we examined the potential benefits of sustained overexpression of the mitogenic and pro-anabolic insulin-like growth factor I (IGF-I) via gene transfer upon the biological activities of human MSCs (hMSCs).
Methods: Recombinant adeno-associated vectors (rAAV) were used to deliver a human IGF-I coding sequence in undifferentiated and chondrogenically-induced primary hMSCs in order to determine the efficacy and duration of transgene expression and the subsequent effects of the genetic modification upon the chondrogenic versus osteogenic differentiation profiles of the cells relative to control (lacZ) treatment after 21 days in vitro.
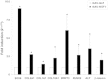
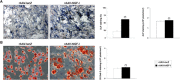
Results: Significant and prolonged expression of IGF-I was evidenced in undifferentiated and most importantly in chondrogenically-induced hMSCs transduced with the candidate rAAV-hIGF-I vector for up to 21 days, leading to enhanced proliferative, biosynthetic, and chondrogenic activities compared with rAAV-lacZ treatment. Overexpression of IGF-I as achieved in the conditions applied here also increased the expression of hypertrophic and osteogenic markers in the treated cells.
Conclusions: These results suggest that a tight regulation of rAAV expression may be necessary for further translation of the approach in clinically relevant animal models in vivo. However, the current findings support the concept of using this type of vector as an effective tool to treat articular cartilage defects via gene- and stem cell-based procedures.
Figures




Comment in
-
Gene- and stem cell-based therapeutics for cartilage regeneration and repair.Stem Cell Res Ther. 2015 Apr 15;6(1):78. doi: 10.1186/s13287-015-0058-5. Stem Cell Res Ther. 2015. PMID: 25884598 Free PMC article.
References
-
- O'Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80:1795–1812. - PubMed
-
- Bentley G, Biant LC, Carrington RW, Akmal M, Goldber A, Williams AM, Skinner JA, Pringle J. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg (Br) 2003;85:223–230. doi: 10.1302/0301-620X.85B2.13543. - DOI - PubMed
-
- Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85-A:185–192. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

