Oxygenase-catalyzed desymmetrization of N,N-dialkyl-piperidine-4-carboxylic acids
- PMID: 25164544
- PMCID: PMC4497603
- DOI: 10.1002/anie.201406125
Oxygenase-catalyzed desymmetrization of N,N-dialkyl-piperidine-4-carboxylic acids
Abstract
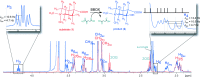
γ-Butyrobetaine hydroxylase (BBOX) is a 2-oxoglutarate dependent oxygenase that catalyzes the final hydroxylation step in the biosynthesis of carnitine. BBOX was shown to catalyze the oxidative desymmetrization of achiral N,N-dialkyl piperidine-4-carboxylates to give products with two or three stereogenic centers.
Keywords: C-H activation; biocatalysis; desymmetrisation; oxygenases; γ-butyrobetaine hydroxylase.
© 2014 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

