Molecular mechanisms contributing to TARP regulation of channel conductance and polyamine block of calcium-permeable AMPA receptors
- PMID: 25164663
- PMCID: PMC4145172
- DOI: 10.1523/JNEUROSCI.0383-14.2014
Molecular mechanisms contributing to TARP regulation of channel conductance and polyamine block of calcium-permeable AMPA receptors
Abstract
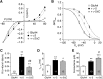
Many properties of fast synaptic transmission in the brain are influenced by transmembrane AMPAR regulatory proteins (TARPs) that modulate the pharmacology and gating of AMPA-type glutamate receptors (AMPARs). Although much is known about TARP influence on AMPAR pharmacology and kinetics through their modulation of the extracellular ligand-binding domain (LBD), less is known about their regulation of the ion channel region. TARP-induced modifications in AMPAR channel behavior include increased single-channel conductance and weakened block of calcium-permeable AMPARs (CP-AMPARs) by endogenous intracellular polyamines. To investigate how TARPs modify ion flux and channel block, we examined the action of γ-2 (stargazin) on GluA1 and GluA4 CP-AMPARs. First, we compared the permeation of organic cations of different sizes. We found that γ-2 increased the permeability of several cations but not the estimated AMPAR pore size, suggesting that TARP-induced relief of polyamine block does not reflect altered pore diameter. Second, to determine whether residues in the TARP intracellular C-tail regulate polyamine block and channel conductance, we examined various γ-2 C-tail mutants. We identified the membrane proximal region of the C terminus as crucial for full TARP-attenuation of polyamine block, whereas complete deletion of the C-tail markedly enhanced the TARP-induced increase in channel conductance; thus, the TARP C-tail influences ion permeation. Third, we identified a site in the pore-lining region of the AMPAR, close to its Q/R site, that is crucial in determining the TARP-induced changes in single-channel conductance. This conserved residue represents a site of TARP action, independent of the AMPAR LBD.
Keywords: AMPA receptors; TARP action; TARPs; calcium-permeable AMPARs; channel conductance; polyamine block.
Copyright © 2014 Soto et al.
Figures



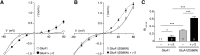

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
