Structures formed by a cell membrane-associated arabinogalactan-protein on graphite or mica alone and with Yariv phenylglycosides
- PMID: 25164699
- PMCID: PMC4195565
- DOI: 10.1093/aob/mcu172
Structures formed by a cell membrane-associated arabinogalactan-protein on graphite or mica alone and with Yariv phenylglycosides
Abstract
Background: Certain membrane-associated arabinogalactan-proteins (AGPs) with lysine-rich sub-domains participate in plant growth, development and resistance to stress. To complement fluorescence imaging of such molecules when tagged and introduced transgenically to the cell periphery and to extend the groundwork for assessing molecular structure, some behaviours of surface-spread AGPs were visualized at the nanometre scale in a simplified electrostatic environment.
Methods: Enhanced green fluorescent protein (EGFP)-labelled LeAGP1 was isolated from Arabidopsis thaliana leaves using antibody-coated magnetic beads, deposited on graphite or mica, and examined with atomic force microscopy (AFM).
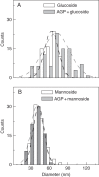
Key results: When deposited at low concentration on graphite, LeAGP can form independent clusters and rings a few nanometres in diameter, often defining deep pits; the aperture of the rings depends on plating parameters. On mica, intermediate and high concentrations, respectively, yielded lacy meshes and solid sheets that could dynamically evolve arcs, rings, 'pores' and 'co-pores', and pits. Glucosyl Yariv reagent combined with the AGP to make very large and distinctive rings.
Conclusions: Diverse cell-specific nano-patterns of native lysine-rich AGPs are expected at the wall-membrane interface and, while there will not be an identical patterning in different environmental settings, AFM imaging suggests protein tendencies for surficial organization and thus opens new avenues for experimentation. Nanopore formation with Yariv reagents suggests how the reagent might bind with AGP to admit Ca(2+) to cells and hints at ways in which AGP might be structured at some cell surfaces.
Keywords: Arabidopsis thaliana; EGFP; LeAGP1; Plant cell walls; Yariv phenylglycoside; atomic force microscopy; glycoprotein cluster; glycoprotein mesh; lysine-rich arabinogalactan-protein; nanopore; periplasm.
© The Author 2014. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures







References
-
- Delcour AH. Function and modulation of bacterial porins: insights from electrophysiology. FEMS Microbiology Letters. 1997;151:115–123. - PubMed
-
- Ding JP, Pickard BG. Mechanosensory calcium-selective cation channels in epidermal cells. The Plant Journal. 1993a;3:83–110. - PubMed
-
- Ding JP, Pickard BG. Modulation of mechanosensitive calcium-selective cation channels by temperature. The Plant Journal. 1993b;3:713–720. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

