Fatigue during treatment for hepatitis C virus: results of self-reported fatigue severity in two Phase IIb studies of simeprevir treatment in patients with hepatitis C virus genotype 1 infection
- PMID: 25164700
- PMCID: PMC4162924
- DOI: 10.1186/1471-2334-14-465
Fatigue during treatment for hepatitis C virus: results of self-reported fatigue severity in two Phase IIb studies of simeprevir treatment in patients with hepatitis C virus genotype 1 infection
Abstract
Background: Fatigue is a common symptom of chronic hepatitis C virus (HCV) infection and a frequent side-effect of peginterferon/ribavirin (PR) therapy for HCV. This study evaluated the impact of adding the oral HCV NS3/4A protease inhibitor simeprevir to PR on patient-reported fatigue and health status among patients with chronic HCV genotype 1 infection enrolled in the Phase IIb PILLAR and ASPIRE trials [NCT00882908; NCT00980330].
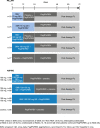
Methods: Treatment-naïve patients (PILLAR, n = 386) and treatment-experienced patients (ASPIRE, n = 462) were randomized to simeprevir plus PR (simeprevir/PR) or placebo plus PR (placebo/PR). In PILLAR, duration of PR treatment in the simeprevir/PR groups was determined using response-guided therapy (RGT) criteria. PR could be terminated at Week 24, instead of Week 48, if HCV RNA was <25 IU/mL by Week 4 and then undetectable at Weeks 12, 16, and 20. In both studies, patients completed the Fatigue Severity Scale (FSS) and EQ-5D quality-of-life questionnaire in their native language at baseline and throughout the studies up until Week 72.
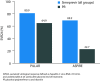
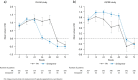
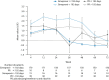
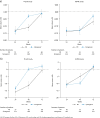
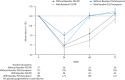
Results: During the first 24 weeks of treatment, mean FSS total score was increased to a similar degree compared with baseline among patients receiving simeprevir/PR or placebo/PR in both studies indicating increased fatigue severity. Mean FSS scores returned to values comparable with baseline among patients receiving simeprevir/PR after Week 24 in PILLAR (after treatment completion for the majority of patients) and in ASPIRE (after Week 48), consistent with RGT enabling early termination of all treatment at Week 24 in 82.2% of simeprevir/PR-treated patients in the PILLAR study. Similar results were observed for EQ-5D, with simeprevir/PR-treated patients experiencing less time with worse health problems according to EQ-5D scores compared with placebo/PR groups in both studies, and more rapid improvement in health status associated with shorter treatment duration in the PILLAR study.
Conclusions: Combination of simeprevir with PR did not increase patient-reported fatigue severity or health status impairments beyond that reported by patients treated with PR alone. Many patients treated with simeprevir/PR returned to pretreatment fatigue and health status levels sooner due to increased treatment efficacy that enabled shorter duration of all therapy, compared with PR alone.
Figures
References
-
- World Health Organization: 63rd World Health Assembly. Provisional agenda item 11_12; 25 March 2010. http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_15-en.pdf,
-
- Conrad S, Garrett LE, Cooksley WG, Dunne MP, MacDonald GA. Living with chronic hepatitis C means ‘you just haven’t got a normal life any more’. Chronic Illn. 2006;2:121–131. - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2334/14/465/prepub
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

