Lack of interleukin-10-mediated anti-inflammatory signals and upregulated interferon gamma production are linked to increased intestinal epithelial cell apoptosis in pathogenic simian immunodeficiency virus infection
- PMID: 25165117
- PMCID: PMC4249077
- DOI: 10.1128/JVI.01757-14
Lack of interleukin-10-mediated anti-inflammatory signals and upregulated interferon gamma production are linked to increased intestinal epithelial cell apoptosis in pathogenic simian immunodeficiency virus infection
Abstract
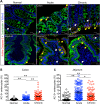
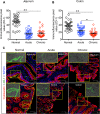
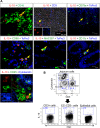
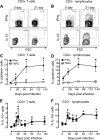
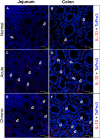
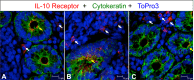
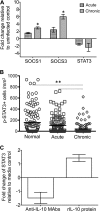
Interleukin-10 (IL-10) is an immunomodulatory cytokine that is important for maintenance of epithelial cell (EC) survival and anti-inflammatory responses (AIR). The majority of HIV infections occur through the mucosal route despite mucosal epithelium acting as a barrier to human immunodeficiency virus (HIV). Therefore, understanding the role of IL-10 in maintenance of intestinal homeostasis during HIV infection is of interest for better characterization of the pathogenesis of HIV-mediated enteropathy. We demonstrated here changes in mucosal IL-10 signaling during simian immunodeficiency virus (SIV) infection in rhesus macaques. Disruption of the epithelial barrier was manifested by EC apoptosis and loss of the tight-junction protein ZO-1. Multiple cell types, including a limited number of ECs, produced IL-10. SIV infection resulted in increased levels of IL-10; however, this was associated with increased production of mucosal gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), suggesting that IL-10 was not able to regulate AIR. This observation was supported by the downregulation of STAT3, which is necessary to inhibit production of IFN-γ and TNF-α, and the upregulation of SOCS1 and SOCS3, which are important regulatory molecules in the IL-10-mediated AIR. We also observed internalization of the IL-10 receptor (IL-10R) in mucosal lymphocytes, which could limit cellular availability of IL-10 for signaling and contribute to the loss of a functional AIR. Collectively, these findings demonstrate that internalization of IL-10R with the resultant impact on IL-10 signaling and dysregulation of the IL-10-mediated AIR might play a crucial role in EC damage and subsequent SIV/HIV pathogenesis.
Importance: Interleukin-10 (IL-10), an important immunomodulatory cytokine plays a key role to control inflammatory function and homeostasis of the gastrointestinal mucosal immune system. Despite recent advancements in the study of IL-10 and its role in HIV infection, the role of mucosal IL-10 in SIV/HIV infection in inducing enteropathy is not well understood. We demonstrated changes in mucosal IL-10 signaling during SIV infection in rhesus macaques. Disruption of the intestinal epithelial barrier was evident along with the increased levels of mucosal IL-10 production. Increased production of mucosal IFN-γ and TNF-α during SIV infection suggested that the increased level of mucosal IL-10 was not able to regulate anti-inflammatory responses. Our findings demonstrate that internalization of IL-10R with the resultant impact on IL-10 signaling and dysregulation of the IL-10-mediated anti-inflammatory responses might play a crucial role in epithelial cell damage and subsequent SIV/HIV pathogenesis.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Batman PA, Kapembwa MS, Miller AR, Sedgwick PM, Lucas S, Sewankambo NK, Serwadda D, Pudney J, Moody A, Harris JR, Griffin GE. 1998. HIV enteropathy: comparative morphometry of the jejunal mucosa of HIV-infected patients resident in the United Kingdom and Uganda. Gut 43:350–355. 10.1136/gut.43.3.350. - DOI - PMC - PubMed
-
- Lim SG, Menzies IS, Lee CA, Johnson MA, Pounder RE. 1993. Intestinal permeability and function in patients infected with human immunodeficiency virus: a comparison with coeliac disease. Scand. J. Gastroenterol. 28:573–580. - PubMed
-
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365–1371. - PubMed
-
- Sankaran S, George MD, Reay E, Guadalupe M, Flamm J, Prindiville T, Dandekar S. 2008. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J. Virol. 82:538–545. 10.1128/JVI.01449-07. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

