Single-molecule tracking of tau reveals fast kiss-and-hop interaction with microtubules in living neurons
- PMID: 25165145
- PMCID: PMC4230615
- DOI: 10.1091/mbc.E14-06-1099
Single-molecule tracking of tau reveals fast kiss-and-hop interaction with microtubules in living neurons
Abstract
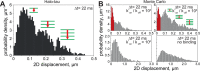
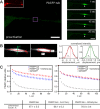
The microtubule-associated phosphoprotein tau regulates microtubule dynamics and is involved in neurodegenerative diseases collectively called tauopathies. It is generally believed that the vast majority of tau molecules decorate axonal microtubules, thereby stabilizing them. However, it is an open question how tau can regulate microtubule dynamics without impeding microtubule-dependent transport and how tau is also available for interactions other than those with microtubules. Here we address this apparent paradox by fast single-molecule tracking of tau in living neurons and Monte Carlo simulations of tau dynamics. We find that tau dwells on a single microtubule for an unexpectedly short time of ∼40 ms before it hops to the next. This dwell time is 100-fold shorter than previously reported by ensemble measurements. Furthermore, we observed by quantitative imaging using fluorescence decay after photoactivation recordings of photoactivatable GFP-tagged tubulin that, despite this rapid dynamics, tau is capable of regulating the tubulin-microtubule balance. This indicates that tau's dwell time on microtubules is sufficiently long to influence the lifetime of a tubulin subunit in a GTP cap. Our data imply a novel kiss-and-hop mechanism by which tau promotes neuronal microtubule assembly. The rapid kiss-and-hop interaction explains why tau, although binding to microtubules, does not interfere with axonal transport.
© 2014 Janning, Igaev, et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures





References
-
- Bakota L, Brandt R, Heinisch JJ. Triple mammalian/yeast/bacterial shuttle vectors for single and combined Lentivirus-and Sindbis virus-mediated infections of neurons. Mol Genet Genomics. 2012;287:313–324. - PubMed
-
- Ballatore C, Lee VM-Y, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. - PubMed
-
- Bates DM, Watts DG. Nonlinear Regression Analysis and Its Applications. New York: Wiley; 1988.
-
- Brandt R, Lee G. Functional organization of microtubule-associated protein tau. Identification of regions which affect microtubule growth, nucleation, and bundle formation in vitro. J Biol Chem. 1993a;268:3414–3419. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

