Rabies Virus Hijacks and accelerates the p75NTR retrograde axonal transport machinery
- PMID: 25165859
- PMCID: PMC4148448
- DOI: 10.1371/journal.ppat.1004348
Rabies Virus Hijacks and accelerates the p75NTR retrograde axonal transport machinery
Abstract
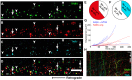
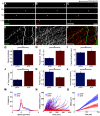
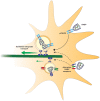
Rabies virus (RABV) is a neurotropic virus that depends on long distance axonal transport in order to reach the central nervous system (CNS). The strategy RABV uses to hijack the cellular transport machinery is still not clear. It is thought that RABV interacts with membrane receptors in order to internalize and exploit the endosomal trafficking pathway, yet this has never been demonstrated directly. The p75 Nerve Growth Factor (NGF) receptor (p75NTR) binds RABV Glycoprotein (RABV-G) with high affinity. However, as p75NTR is not essential for RABV infection, the specific role of this interaction remains in question. Here we used live cell imaging to track RABV entry at nerve terminals and studied its retrograde transport along the axon with and without the p75NTR receptor. First, we found that NGF, an endogenous p75NTR ligand, and RABV, are localized in corresponding domains along nerve tips. RABV and NGF were internalized at similar time frames, suggesting comparable entry machineries. Next, we demonstrated that RABV could internalize together with p75NTR. Characterizing RABV retrograde movement along the axon, we showed the virus is transported in acidic compartments, mostly with p75NTR. Interestingly, RABV is transported faster than NGF, suggesting that RABV not only hijacks the transport machinery but can also manipulate it. Co-transport of RABV and NGF identified two modes of transport, slow and fast, that may represent a differential control of the trafficking machinery by RABV. Finally, we determined that p75NTR-dependent transport of RABV is faster and more directed than p75NTR-independent RABV transport. This fast route to the neuronal cell body is characterized by both an increase in instantaneous velocities and fewer, shorter stops en route. Hence, RABV may employ p75NTR-dependent transport as a fast mechanism to facilitate movement to the CNS.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Salinas S, Schiavo G, Kremer EJ (2010) A hitchhiker's guide to the nervous system: the complex journey of viruses and toxins. Nat Rev Microbiol 8: 645–655 Available: http://www.ncbi.nlm.nih.gov/pubmed/20706281. Accessed 27 January 2014. - PubMed
-
- Zampieri N, Jessell TM, Murray AJ (2014) Mapping Sensory Circuits by Anterograde Transsynaptic Transfer of Recombinant Rabies Virus. Neuron 1–13 Available: http://www.ncbi.nlm.nih.gov/pubmed/24486087. Accessed 4 February 2014. - PMC - PubMed
-
- Ugolini G (2008) Use of rabies virus as a transneuronal tracer of neuronal connections: implications for the understanding of rabies pathogenesis. Dev Biol (Basel) 131: 493–506 Available: http://www.ncbi.nlm.nih.gov/pubmed/18634512. Accessed 11 March 2014. - PubMed
-
- Wickersham IR, Finke S, Conzelmann K-K, Callaway EM (2007) Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat Methods 4: 47–49 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2755236&tool=p.... Accessed 27 January 2014. - PMC - PubMed
-
- Wickersham IR, Lyon DC, Barnard RJO, Mori T, Finke S, et al. (2007) Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53: 639–647 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2629495&tool=p.... Accessed 21 January 2014. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

