Efficient strategy for the molecular diagnosis of intellectual disability using targeted high-throughput sequencing
- PMID: 25167861
- PMCID: PMC4215287
- DOI: 10.1136/jmedgenet-2014-102554
Efficient strategy for the molecular diagnosis of intellectual disability using targeted high-throughput sequencing
Abstract
Background: Intellectual disability (ID) is characterised by an extreme genetic heterogeneity. Several hundred genes have been associated to monogenic forms of ID, considerably complicating molecular diagnostics. Trio-exome sequencing was recently proposed as a diagnostic approach, yet remains costly for a general implementation.
Methods: We report the alternative strategy of targeted high-throughput sequencing of 217 genes in which mutations had been reported in patients with ID or autism as the major clinical concern. We analysed 106 patients with ID of unknown aetiology following array-CGH analysis and other genetic investigations. Ninety per cent of these patients were males, and 75% sporadic cases.
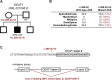
Results: We identified 26 causative mutations: 16 in X-linked genes (ATRX, CUL4B, DMD, FMR1, HCFC1, IL1RAPL1, IQSEC2, KDM5C, MAOA, MECP2, SLC9A6, SLC16A2, PHF8) and 10 de novo in autosomal-dominant genes (DYRK1A, GRIN1, MED13L, TCF4, RAI1, SHANK3, SLC2A1, SYNGAP1). We also detected four possibly causative mutations (eg, in NLGN3) requiring further investigations. We present detailed reasoning for assigning causality for each mutation, and associated patients' clinical information. Some genes were hit more than once in our cohort, suggesting they correspond to more frequent ID-associated conditions (KDM5C, MECP2, DYRK1A, TCF4). We highlight some unexpected genotype to phenotype correlations, with causative mutations being identified in genes associated to defined syndromes in patients deviating from the classic phenotype (DMD, TCF4, MECP2). We also bring additional supportive (HCFC1, MED13L) or unsupportive (SHROOM4, SRPX2) evidences for the implication of previous candidate genes or mutations in cognitive disorders.
Conclusions: With a diagnostic yield of 25% targeted sequencing appears relevant as a first intention test for the diagnosis of ID, but importantly will also contribute to a better understanding regarding the specific contribution of the many genes implicated in ID and autism.
Keywords: autism; causative; high-throughput sequencing; intellectual disability; mutation.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures


References
-
- Leonard H, Wen X. The epidemiology of mental retardation: challenges and opportunities in the new millennium. Ment Retard Dev Disabil Res Rev 2002;8:117–34. - PubMed
-
- Maulik PK, Mascarenhas MN, Mathers CD, Dua T, Saxena S. Prevalence of intellectual disability: a meta-analysis of population-based studies. Res Dev Disabil 2011;32:419–36. - PubMed
-
- de Ligt J, Willemsen MH, van Bon BW, Kleefstra T, Yntema HG, Kroes T, Vulto-van Silfhout AT, Koolen DA, de Vries P, Gilissen C, del Rosario M, Hoischen A, Scheffer H, de Vries BB, Brunner HG, Veltman JA, Vissers LE. Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med 2012;367:1921–9. - PubMed
-
- Najmabadi H, Hu H, Garshasbi M, Zemojtel T, Abedini SS, Chen W, Hosseini M, Behjati F, Haas S, Jamali P, Zecha A, Mohseni M, Puttmann L, Vahid LN, Jensen C, Moheb LA, Bienek M, Larti F, Mueller I, Weissmann R, Darvish H, Wrogemann K, Hadavi V, Lipkowitz B, Esmaeeli-Nieh S, Wieczorek D, Kariminejad R, Firouzabadi SG, Cohen M, Fattahi Z, Rost I, Mojahedi F, Hertzberg C, Dehghan A, Rajab A, Banavandi MJ, Hoffer J, Falah M, Musante L, Kalscheuer V, Ullmann R, Kuss AW, Tzschach A, Kahrizi K, Ropers HH. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature 2011;478:57–63. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
