Autophagy in tuberculosis
- PMID: 25167980
- PMCID: PMC4208715
- DOI: 10.1101/cshperspect.a018481
Autophagy in tuberculosis
Abstract
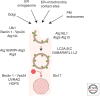
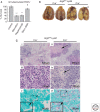
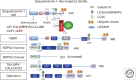
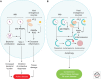
Autophagy as an immune mechanism controls inflammation and acts as a cell-autonomous defense against intracellular microbes including Mycobacterium tuberculosis. An equally significant role of autophagy is its anti-inflammatory and tissue-sparing function. This combination of antimicrobial and anti-inflammatory actions prevents active disease in animal models. In human populations, genetic links between autophagy, inflammatory bowel disease, and susceptibility to tuberculosis provide further support to these combined roles of autophagy. The autophagic control of M. tuberculosis and prevention of progressive disease provide novel insights into physiological and immune control of tuberculosis. It also offers host-based therapeutic opportunities because autophagy can be pharmacologically modulated.
Copyright © 2014 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
-
- Anandaiah A, Sinha S, Bole M, Sharma SK, Kumar N, Luthra K, Li X, Zhou X, Nelson B, Han X, et al. 2013. Vitamin D rescues impaired Mycobacterium tuberculosis–mediated tumor necrosis factor release in macrophages of HIV-seropositive individuals through an enhanced Toll-like receptor signaling pathway in vitro. Infect Immun 81: 2–10 - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
