One-pot synthesis of manganese oxide-carbon composite microspheres with three dimensional channels for Li-ion batteries
- PMID: 25168839
- PMCID: PMC5385819
- DOI: 10.1038/srep05751
One-pot synthesis of manganese oxide-carbon composite microspheres with three dimensional channels for Li-ion batteries
Abstract
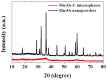
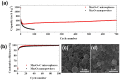
The fabrication of manganese oxide-carbon composite microspheres with open nanochannels and their electrochemical performance as anode materials for lithium ion batteries are investigated. Amorphous-like Mn3O4 nanoparticles embedded in a carbon matrix with three-dimensional channels are fabricated by one-pot spray pyrolysis. The electrochemical properties of the Mn3O4 nanopowders are also compared with those of the Mn3O4-C composite microspheres possessing macropores resembling ant-cave networks. The discharge capacity of the Mn3O4-C composite microspheres at a current density of 500 mA g(-1) is 622 mA h g(-1) after 700 cycles. However, the discharge capacity of the Mn3O4 nanopowders is as low as 219 mA h g(-1) after 100 cycles. The Mn3O4-C composite microspheres with structural advantages and high electrical conductivity have higher initial discharge and charge capacities and better cycling and rate performances compared to those of the Mn3O4 nanopowders.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
One-pot facile synthesis of ant-cave-structured metal oxide-carbon microballs by continuous process for use as anode materials in Li-ion batteries.Nano Lett. 2013;13(11):5462-6. doi: 10.1021/nl4030352. Epub 2013 Oct 24. Nano Lett. 2013. PMID: 24144195
-
Large Scale Process for Low Crystalline MoO₃-Carbon Composite Microspheres Prepared by One-Step Spray Pyrolysis for Anodes in Lithium-Ion Batteries.Nanomaterials (Basel). 2019 Apr 3;9(4):539. doi: 10.3390/nano9040539. Nanomaterials (Basel). 2019. PMID: 30987189 Free PMC article.
-
One-pot rapid synthesis of core-shell structured NiO@TiO2 nanopowders and their excellent electrochemical properties as anode materials for lithium ion batteries.Nanoscale. 2013 Dec 21;5(24):12645-50. doi: 10.1039/c3nr04406h. Nanoscale. 2013. PMID: 24177597
-
Engineering single crystalline Mn3O4 nano-octahedra with exposed highly active {011} facets for high performance lithium ion batteries.Nanoscale. 2014 Jun 21;6(12):6819-27. doi: 10.1039/c4nr01389a. Nanoscale. 2014. PMID: 24828316
-
Carbon nanoparticle-entrapped macroporous Mn3O4 microsphere anodes with improved cycling stability for Li-ion batteries.Sci Rep. 2022 Jul 14;12(1):11992. doi: 10.1038/s41598-022-16383-0. Sci Rep. 2022. PMID: 35835846 Free PMC article.
Cited by
-
Pt-free, low-cost and efficient counter electrode with carbon wrapped VO2(M) nanofiber for dye-sensitized solar cells.Sci Rep. 2019 Mar 26;9(1):5177. doi: 10.1038/s41598-019-41693-1. Sci Rep. 2019. PMID: 30914740 Free PMC article.
-
Co-Existence of Iron Oxide Nanoparticles and Manganese Oxide Nanorods as Decoration of Hollow Carbon Spheres for Boosting Electrochemical Performance of Li-Ion Battery.Materials (Basel). 2021 Nov 15;14(22):6902. doi: 10.3390/ma14226902. Materials (Basel). 2021. PMID: 34832303 Free PMC article.
-
Modulation of miRNA-155 alters manganese nanoparticle-induced inflammatory response.Toxicol Res (Camb). 2016 Oct 13;5(6):1733-1743. doi: 10.1039/c6tx00208k. eCollection 2016 Nov 1. Toxicol Res (Camb). 2016. PMID: 30090472 Free PMC article.
References
-
- Poizot P., Laruelle S., Grugeon S., Dupont L. & Tarascon J. M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 407, 496–499 (2000). - PubMed
-
- Reddy M. V., Subba Rao G. V. & Chowdari B. V. R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 113, 5364–5457 (2013). - PubMed
-
- Poizot P., Laruelle S., Grugeon S., Dupont L. & Tarascon J. M. Searching for new anode materials for the Li-ion technology: time to device from the usual path. J. Power Sources 97–98, 235–239 (2001).
-
- Reddy A. L. M., Shaijumon M. M., Gowda S. R. & Ajayan P. M. Coaxial MnO2/carbon nanotube array electrodes for high-performance lithium batteries. Nano Lett. 9, 1002–1006 (2009). - PubMed
-
- Chen J. S. & Lou X. W. SnO2-based nanomaterials: synthesis and application in lithium-ion batteries. Small 9, 1877–1893 (2013). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

