Development of an antifungal denture adhesive film for oral candidiasis utilizing hot melt extrusion technology
- PMID: 25169007
- PMCID: PMC5629914
- DOI: 10.1517/17425247.2014.949235
Development of an antifungal denture adhesive film for oral candidiasis utilizing hot melt extrusion technology
Abstract
Objectives: The overall goal of this research was to produce a stable hot-melt extruded 'Antifungal Denture Adhesive film' (ADA) system for the treatment of oral candidiasis.
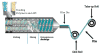
Methods: The ADA systems with hydroxypropyl cellulose (HPC) and/or polyethylene oxide (PEO) containing clotrimazole (10%) or nystatin (10%) were extruded utilizing a lab scale twin-screw hot-melt extruder. Rolls of the antifungal-containing films were collected and subsequently die-cut into shapes adapted for a maxillary (upper) and mandibular (lower) denture.
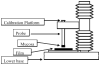
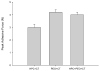
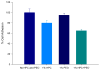
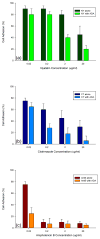
Results: Differential scanning calorimeter and powder X-ray diffraction results indicated that the crystallinity of both APIs was changed to amorphous phase after hot-melt extrusion. The ADA system, containing blends of HPC and PEO, enhanced the effectiveness of the antimicrobials a maximum of fivefold toward the inhibition of cell adherence of Candida albicans to mammalian cells/Vero cells. Remarkably, a combination of the two polymers without drug also demonstrated a 38% decrease in cell adhesion to the fungi due to the viscosity and the flexibility of the polymers. Drug-release profiles indicated that both drug concentrations were above the minimum inhibitory concentration (MIC) for C. albicans within 10 min and was maintained for over 10 h. In addition, based on the IC50 and MIC values, it was observed that the antifungal activities of both drugs were increased significantly in the ADA systems.
Conclusions: Based on these findings, the ADA system may be used for primary, prophylaxis or adjunct treatment of oral or pharyngeal candidiasis via controlled release of the antifungal agent from the polymer matrix.
Keywords: bioadhesive film; hot-melt extrusion; hydroxypropyl cellulose; oral candidiasis; polyethylene oxide.
Conflict of interest statement
This project was funded by Grant Numbers P20GM104932 and R43DE15455-01 from the National Institute of General Medical Sciences (NIGMS) and NIDCR, respectfully, components of NIH for contributions to this project.
Figures






Similar articles
-
Stabilization of hot-melt extrusion formulations containing solid solutions using polymer blends.AAPS PharmSciTech. 2007 Jun 29;8(2):Article 50. doi: 10.1208/pt0802050. AAPS PharmSciTech. 2007. PMID: 17622123 Free PMC article.
-
Production and characterization of hot-melt extruded films containing clotrimazole.Drug Dev Ind Pharm. 2003 Aug;29(7):757-65. doi: 10.1081/ddc-120021775. Drug Dev Ind Pharm. 2003. PMID: 12906333
-
Clotrimazole mucoadhesive films with extended-release properties for vaginal candidiasis-A hot-melt extrusion application.J Pharm Sci. 2025 Feb;114(2):1296-1306. doi: 10.1016/j.xphs.2025.01.011. Epub 2025 Jan 16. J Pharm Sci. 2025. PMID: 39826839
-
Antimycotic agents in oral candidosis: an overview: 2. Treatment of oral candidosis.Dent Update. 2000 May;27(4):165-70, 172-4. doi: 10.12968/denu.2000.27.4.165. Dent Update. 2000. PMID: 11218451 Review.
-
Cutaneous candidiasis - an evidence-based review of topical and systemic treatments to inform clinical practice.J Eur Acad Dermatol Venereol. 2019 Oct;33(10):1863-1873. doi: 10.1111/jdv.15782. Epub 2019 Jul 30. J Eur Acad Dermatol Venereol. 2019. PMID: 31287594 Review.
Cited by
-
Chlorhexidine Mucoadhesive Buccal Tablets: The Impact of Formulation Design on Drug Delivery and Release Kinetics Using Conventional and Novel Dissolution Methods.Pharmaceuticals (Basel). 2021 May 23;14(6):493. doi: 10.3390/ph14060493. Pharmaceuticals (Basel). 2021. PMID: 34070990 Free PMC article.
-
In Vitro and In Vivo Investigation of a Dual-Targeted Nanoemulsion Gel for the Amelioration of Psoriasis.Gels. 2023 Jan 28;9(2):112. doi: 10.3390/gels9020112. Gels. 2023. PMID: 36826282 Free PMC article.
-
Development of a Solid Dispersion of Nystatin with Maltodextrin as a Carrier Agent: Improvements in Antifungal Efficacy against Candida spp. Biofilm Infections.Pharmaceuticals (Basel). 2021 Apr 22;14(5):397. doi: 10.3390/ph14050397. Pharmaceuticals (Basel). 2021. PMID: 33922089 Free PMC article.
-
Technological Aspects and Evaluation Methods for Polymer Matrices as Dental Drug Carriers.Biomedicines. 2023 Apr 25;11(5):1274. doi: 10.3390/biomedicines11051274. Biomedicines. 2023. PMID: 37238944 Free PMC article. Review.
References
-
- Vazquez JA. Options for the management of mucosal candidiasis in patients with AIDS and HIV infection. Pharmacotherapy. 1999;19(1):76–87. - PubMed
-
- IMS. IMS America. 2009. Data on oral and pharyngeal candidiasis.
-
- Abu-Elteen K, Abu-Alteen R. The prevalence of Candida albicans populations in the mouths of complete denture wearers. New Microbiol. 1998;21(1):41–48. - PubMed
-
- Epstein JB, Polsky B. Oropharyngeal candidiasis: a review of its clinical spectrum and current therapies. Clin Ther. 1998;20(1):40–57. This article reviewed higher risks for oral fungal opportunistic infections. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
