Control of synaptic function by endocannabinoid-mediated retrograde signaling
- PMID: 25169670
- PMCID: PMC4237895
- DOI: 10.2183/pjab.90.235
Control of synaptic function by endocannabinoid-mediated retrograde signaling
Abstract
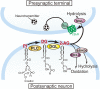
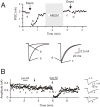
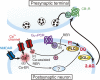
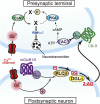
Since the first reports in 2001, great advances have been made towards the understanding of endocannabinoid-mediated synaptic modulation. Electrophysiological studies have revealed that one of the two major endocannabinoids, 2-arachidonoylglycerol (2-AG), is produced from membrane lipids upon postsynaptic Ca(2+) elevation and/or activation of Gq/11-coupled receptors, and released from postsynaptic neurons. The released 2-AG then acts retrogradely onto presynaptic cannabinoid CB1 receptors and induces suppression of neurotransmitter release either transiently or persistently. These forms of 2-AG-mediated retrograde synaptic modulation are functional throughout the brain. The other major endocannabinoid, anandamide, mediates a certain form of endocannabinoid-mediated long-term depression (LTD). Anandamide also functions as an agonist for transient receptor potential vanilloid receptor type 1 (TRPV1) and mediates endocannabinoid-independent and TRPV1-dependent forms of LTD. It has also been demonstrated that the endocannabinoid system itself is plastic, which can be either up- or down-regulated by experimental or environmental conditions. In this review, I will make an overview of the mechanisms underlying endocannabinoid-mediated synaptic modulation.
Figures
Similar articles
-
Endocannabinoid-mediated retrograde modulation of synaptic transmission.Curr Opin Neurobiol. 2014 Dec;29:1-8. doi: 10.1016/j.conb.2014.03.017. Epub 2014 Apr 18. Curr Opin Neurobiol. 2014. PMID: 24747340 Review.
-
Calcium signaling and synaptic modulation: regulation of endocannabinoid-mediated synaptic modulation by calcium.Cell Calcium. 2005 Sep-Oct;38(3-4):369-74. doi: 10.1016/j.ceca.2005.06.014. Cell Calcium. 2005. PMID: 16085309 Review.
-
[Lipid mediator endocannabinoids in modulating synaptic transmission].Nihon Shinkei Seishin Yakurigaku Zasshi. 2011 Jun;31(3):105-9. Nihon Shinkei Seishin Yakurigaku Zasshi. 2011. PMID: 21800700 Review. Japanese.
-
Endocannabinoid-dependent LTD in a nociceptive synapse requires activation of a presynaptic TRPV-like receptor.J Neurophysiol. 2010 Nov;104(5):2766-77. doi: 10.1152/jn.00491.2010. Epub 2010 Sep 8. J Neurophysiol. 2010. PMID: 20884761 Free PMC article.
-
Roles of phospholipase Cbeta and NMDA receptor in activity-dependent endocannabinoid release.J Physiol. 2007 Oct 15;584(Pt 2):373-80. doi: 10.1113/jphysiol.2007.137497. Epub 2007 Jul 5. J Physiol. 2007. PMID: 17615097 Free PMC article.
Cited by
-
2-AG and anandamide enhance hippocampal long-term potentiation via suppression of inhibition.Front Cell Neurosci. 2022 Sep 21;16:1023541. doi: 10.3389/fncel.2022.1023541. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36212685 Free PMC article.
-
Modulation of pulmonary immune function by inhaled cannabis products and consequences for lung disease.Respir Res. 2023 Mar 28;24(1):95. doi: 10.1186/s12931-023-02399-1. Respir Res. 2023. PMID: 36978106 Free PMC article. Review.
-
Cell-Autonomous Excitation of Midbrain Dopamine Neurons by Endocannabinoid-Dependent Lipid Signaling.Neuron. 2017 Mar 22;93(6):1375-1387.e2. doi: 10.1016/j.neuron.2017.02.025. Epub 2017 Mar 2. Neuron. 2017. PMID: 28262417 Free PMC article.
-
Cellular Mechanisms of Action of Drug Abuse on Olfactory Neurons.Int J Environ Res Public Health. 2015 Dec 22;13(1):ijerph13010005. doi: 10.3390/ijerph13010005. Int J Environ Res Public Health. 2015. PMID: 26703658 Free PMC article. Review.
-
Endocannabinoid system in psychotic and mood disorders, a review of human studies.Prog Neuropsychopharmacol Biol Psychiatry. 2021 Mar 2;106:110096. doi: 10.1016/j.pnpbp.2020.110096. Epub 2020 Sep 6. Prog Neuropsychopharmacol Biol Psychiatry. 2021. PMID: 32898588 Free PMC article. Review.
References
-
- Iversen, L. (2000) The Science of Marijuana. Oxford University Press, Inc., New York.
-
- Katzung, B.G., Masters, S.B. and Trevor, A.J. (2009) Basic and Clinical Pharmacology. MaGraw-Hill Companies, Inc., New York.
-
- Gaoni Y., Mechoulam R. (1964) Isolation, structure and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 86, 1646–1647.
-
- Matsuda L.A., Lolait S.J., Brownstein M.J., Young A.C., Bonner T.I. (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564. - PubMed
-
- Munro S., Thomas K.L., Abu-Shaar M. (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

