Mechanistic interpretation of conventional Michaelis-Menten parameters in a transporter system
- PMID: 25169756
- PMCID: PMC4204736
- DOI: 10.1016/j.ejps.2014.08.007
Mechanistic interpretation of conventional Michaelis-Menten parameters in a transporter system
Abstract
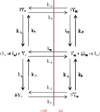
The aim was to elucidate how steps in drug translocation by a solute carrier transporter impact Michaelis-Menten parameters Km, Ki, and Vmax. The first objective was to derive a model for carrier-mediated substrate translocation and perform sensitivity analysis with regard to the impact of individual microrate constants on Km, Ki, and Vmax. The second objective was to compare underpinning microrate constants between compounds translocated by the same transporter. Equations for Km, Ki, and Vmax were derived from a six-state model involving unidirectional transporter flipping and reconfiguration. This unidirectional model is applicable to co-transporter type solute carriers, like the apical sodium-dependent bile acid transporter (ASBT) and the proton-coupled peptide cotransporter (PEPT1). Sensitivity analysis identified the microrate constants that impacted Km, Ki, and Vmax. Compound comparison using the six-state model employed regression to identify microrate constant values that can explain observed Km and Vmax values. Results yielded some expected findings, as well as some unanticipated effects of microrate constants on Km, Ki, and Vmax. Km and Ki were found to be equal for inhibitors that are also substrates. Additionally, microrate constant values for certain steps in transporter functioning influenced Km and Vmax to be low or high.
Keywords: Apical sodium-dependent bile acid transporter; Michaelis–Menten; Model; Transporter.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Bidirectional electrogenic transport of peptides by the proton-coupled carrier PEPT1 in Xenopus laevis oocytes: its asymmetry and symmetry.J Physiol. 2001 Oct 15;536(Pt 2):495-503. doi: 10.1111/j.1469-7793.2001.0495c.xd. J Physiol. 2001. PMID: 11600684 Free PMC article.
-
Functional characterization of brain peptide transporter in rat cerebral cortex: identification of the high-affinity type H+/peptide transporter PEPT2.Brain Res. 2004 Jan 30;997(1):52-61. doi: 10.1016/j.brainres.2003.10.049. Brain Res. 2004. PMID: 14715149
-
Identification of a region of the ileal-type sodium/bile acid cotransporter interacting with a competitive bile acid transport inhibitor.Biochemistry. 2002 Dec 17;41(50):14916-24. doi: 10.1021/bi0205404. Biochemistry. 2002. PMID: 12475240
-
The intestinal H+/peptide symporter PEPT1: structure-affinity relationships.Eur J Pharm Sci. 2004 Jan;21(1):53-60. doi: 10.1016/s0928-0987(03)00142-8. Eur J Pharm Sci. 2004. PMID: 14706811 Review.
-
Di/tri-peptide transporters as drug delivery targets: regulation of transport under physiological and patho-physiological conditions.Curr Drug Targets. 2003 Jul;4(5):373-88. doi: 10.2174/1389450033491028. Curr Drug Targets. 2003. PMID: 12816347 Review.
Cited by
-
High-Throughput Assessment of Bile Salt Export Pump Inhibition Using RapidFire-MS and DESI-MS.ACS Med Chem Lett. 2024 Aug 12;15(9):1584-1590. doi: 10.1021/acsmedchemlett.4c00302. eCollection 2024 Sep 12. ACS Med Chem Lett. 2024. PMID: 39291028
-
Comparative analysis of STP6 and STP10 unravels molecular selectivity in sugar transport proteins.Proc Natl Acad Sci U S A. 2025 Apr 29;122(17):e2417370122. doi: 10.1073/pnas.2417370122. Epub 2025 Apr 25. Proc Natl Acad Sci U S A. 2025. PMID: 40279393
-
Homology modeling and site-directed mutagenesis identify amino acid residues underlying the substrate selection mechanism of human monocarboxylate transporters 1 (hMCT1) and 4 (hMCT4).Cell Mol Life Sci. 2019 Dec;76(24):4905-4921. doi: 10.1007/s00018-019-03151-z. Epub 2019 May 17. Cell Mol Life Sci. 2019. PMID: 31101938 Free PMC article.
-
Direct coupling of oligomerization and oligomerization-driven endocytosis of the dopamine transporter to its conformational mechanics and activity.J Biol Chem. 2021 Jan-Jun;296:100430. doi: 10.1016/j.jbc.2021.100430. Epub 2021 Feb 18. J Biol Chem. 2021. PMID: 33610553 Free PMC article.
-
Synthesis and in vitro evaluation of bile acid prodrugs of floxuridine to target the liver.Int J Pharm. 2014 Nov 20;475(1-2):597-604. doi: 10.1016/j.ijpharm.2014.09.014. Epub 2014 Sep 16. Int J Pharm. 2014. PMID: 25219859 Free PMC article.
References
-
- Garrett R, Grisham C, editors. Biochemistry. 3rd ed. Belmont (CA): Thomson Brooks/Cole; 2005.
-
- Dirr H, Reinemer P, Huber R. X-ray crystal structures of cytosolic glutathione S-transferases: Implications for protein architecture, substrate recognition and catalytic function. Eur. J Biochem. 1994;220:645–661. - PubMed
-
- Sztajer H, Gamain B, Aumann K, Slomianny C, Becker K, Brigelius-Flohé R, Flohé L. The putative glutathione peroxidase gene of Plasmodium falciparum codes for a thioredoxin peroxidase. J Biol Chem. 2001;276:7397–7403. - PubMed
-
- Peet GW, Li J. IκB kinases α and β show a random sequential kinetic mechanism and are inhibited by staurosporine and quercetin. J Biol Chem. 1999;274:32655–32661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

