Exclusion of older patients from ongoing clinical trials for hematological malignancies: an evaluation of the National Institutes of Health Clinical Trial Registry
- PMID: 25170014
- PMCID: PMC4201007
- DOI: 10.1634/theoncologist.2014-0093
Exclusion of older patients from ongoing clinical trials for hematological malignancies: an evaluation of the National Institutes of Health Clinical Trial Registry
Abstract
Introduction: Cancer societies, research cooperatives, and countless publications have urged the development of clinical trials that facilitate the inclusion of older patients and those with comorbidities. We set out to determine the characteristics of currently recruiting clinical trials with hematological patients to assess their inclusion and exclusion of elderly patients.
Methods: The NIH clinical trial registry was searched on July 1, 2013, for currently recruiting phase I, II or III clinical trials with hematological malignancies. Trial characteristics and study objectives were extracted from the registry website.
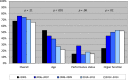
Results: Although 5% of 1,207 included trials focused exclusively on elderly or unfit patients, 69% explicitly or implicitly excluded older patients. Exclusion based on age was seen in 27% of trials, exclusion based on performance status was seen in 16%, and exclusion based on stringent organ function restrictions was noted in 51%. One-third of the studies that excluded older patients based on age allowed inclusion of younger patients with poor performance status; 8% did not place any restrictions on organ function. Over time, there was a shift from exclusion based on age (p value for trend <.001) toward exclusion based on organ function (p = .2). Industry-sponsored studies were least likely to exclude older patients (p < .001).
Conclusion: Notably, 27% of currently recruiting clinical trials for hematological malignancies use age-based exclusion criteria. Although physiological reserves diminish with age, the heterogeneity of the elderly population does not legitimize exclusion based on chronological age alone. Investigators should critically review whether sufficient justification exists for every exclusion criterion before incorporating it in trial protocols.
Keywords: Clinical trial design; Elderly; Exclusion criteria; Hematological malignancies.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
Similar articles
-
Selection of Patients in Ongoing Clinical Trials on Lung Cancer.Lung. 2016 Dec;194(6):967-974. doi: 10.1007/s00408-016-9943-7. Epub 2016 Sep 20. Lung. 2016. PMID: 27650509
-
On-going clinical trials for elderly patients with a hematological malignancy: are we addressing the right end points?Ann Oncol. 2014 Mar;25(3):675-681. doi: 10.1093/annonc/mdt592. Epub 2014 Jan 23. Ann Oncol. 2014. PMID: 24458474 Free PMC article.
-
Exclusion of Acute Myeloid Leukemia Patients with Central Nervous System Involvement from Clinical Trials: An Analysis of the National Institutes of Health Clinical Trials Registry from 2012 to 2022.Acta Haematol. 2024;147(3):292-299. doi: 10.1159/000533819. Epub 2023 Sep 26. Acta Haematol. 2024. PMID: 37751713
-
Innovation in oncology clinical trial design.Cancer Treat Rev. 2019 Mar;74:15-20. doi: 10.1016/j.ctrv.2019.01.001. Epub 2019 Jan 4. Cancer Treat Rev. 2019. PMID: 30665053 Review.
-
Metastatic gastroesophageal cancer in older patients - is this patient cohort represented in clinical trials?BMC Cancer. 2022 Jan 3;22(1):3. doi: 10.1186/s12885-021-09103-w. BMC Cancer. 2022. PMID: 34980003 Free PMC article. Review.
Cited by
-
Olaparib treatment in older patients with ovarian cancer: need for 'real-world' data beyond clinical trials.Ecancermedicalscience. 2020 Sep 15;14:1104. doi: 10.3332/ecancer.2020.1104. eCollection 2020. Ecancermedicalscience. 2020. PMID: 33082854 Free PMC article.
-
A simplified frailty score predicts survival and can aid treatment-intensity decisions in older patients with DLBCL.Blood Adv. 2021 Nov 23;5(22):4771-4782. doi: 10.1182/bloodadvances.2021004777. Blood Adv. 2021. PMID: 34543384 Free PMC article.
-
Comorbidity, Physical Function, and Quality of Life in Older Adults with Acute Myeloid Leukemia.Curr Geriatr Rep. 2017 Dec;6(4):247-254. doi: 10.1007/s13670-017-0227-8. Epub 2017 Oct 11. Curr Geriatr Rep. 2017. PMID: 29479516 Free PMC article.
-
Survival benefit of mantle cell lymphoma patients enrolled in clinical trials; a joint study from the LYSA group and French cancer registries.J Cancer Res Clin Oncol. 2018 Apr;144(4):629-635. doi: 10.1007/s00432-017-2529-9. Epub 2017 Oct 11. J Cancer Res Clin Oncol. 2018. PMID: 29022078 Free PMC article. Clinical Trial.
-
Selection of Patients in Ongoing Clinical Trials on Lung Cancer.Lung. 2016 Dec;194(6):967-974. doi: 10.1007/s00408-016-9943-7. Epub 2016 Sep 20. Lung. 2016. PMID: 27650509
References
-
- Surveillance, Epidemiology, and End Results program. Available at http://seer.cancer.gov Accessed August 14, 2013.
-
- Gundrum DJ, Go RS. Cancer in the oldest old in the United Status: Current statistics and projections. J Geriatr Oncol. 2012;3:299–306.
-
- Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. - PubMed
-
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. - PubMed
-
- Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55:241–252. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases