Infant HIV type 1 gp120 vaccination elicits robust and durable anti-V1V2 immunoglobulin G responses and only rare envelope-specific immunoglobulin A responses
- PMID: 25170104
- PMCID: PMC4318918
- DOI: 10.1093/infdis/jiu444
Infant HIV type 1 gp120 vaccination elicits robust and durable anti-V1V2 immunoglobulin G responses and only rare envelope-specific immunoglobulin A responses
Abstract
Background: Infant responses to vaccines can be impeded by maternal antibodies and immune system immaturity. It is therefore unclear whether human immunodeficiency virus type 1 (HIV-1) vaccination would elicit similar responses in adults and infants.
Method: HIV-1 Env-specific antibody responses were evaluated in 2 completed pediatric vaccine trials. In the Pediatric AIDS Clinical Trials Group (PACTG) 230 protocol, infants were vaccinated with 4 doses of Chiron rgp120 with MF59 (n=48), VaxGen rgp120 with aluminum hydroxide (alum; n=49), or placebo (n=19) between 0 and 20 weeks of age. In PACTG 326, infants received 4 doses of ALVAC-HIV-1/AIDSVAX B/B with alum (n=9) or placebo (n=13) between 0 and 12 weeks of age.
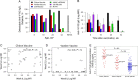
Results: By 52 weeks of age, the majority of maternally acquired antibodies had waned and vaccine Env-specific immunoglobulin G (IgG) responses in vaccinees were higher than in placebo recipients. Chiron vaccine recipients had higher and more-durable IgG responses than VaxGen vaccine recipients or ALVAC/AIDSVAX vaccinees, with vaccine-elicited IgG responses still detectable in 56% of recipients at 2 years of age. Remarkably, at peak immunogenicity, the concentration of anti-V1V2 IgG, a response associated with a reduced risk of HIV-1 acquisition in the RV144 adult vaccine trial, was 22-fold higher in Chiron vaccine recipients, compared with RV144 vaccinees.
Conclusion: As exemplified by the Chiron vaccine regimen, vaccination of infants against HIV-1 can induce robust, durable Env-specific IgG responses, including anti-V1V2 IgG.
Keywords: HIV-1; antibodies; infants; vaccine.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures



Comment in
-
Reevaluating HIV vaccine clinical trials policy for infants.J Infect Dis. 2015 Feb 15;211(4):501-3. doi: 10.1093/infdis/jiu445. Epub 2014 Aug 27. J Infect Dis. 2015. PMID: 25170103 No abstract available.
References
-
- Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–74. - PubMed
-
- UNAIDS. Global report. UNAIDS report on the global AIDS epidemics. 2013. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiolo.... Accessed 12 March 2014.
-
- De Cock KM, Fowler MG, Mercier E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA. 2000;283:1175–82. - PubMed
-
- Siegrist CA. The challenges of vaccine responses in early life: selected examples. J Comp Pathol. 2007;137(suppl 1):S4–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AI64518/AI/NIAID NIH HHS/United States
- UM1 AI100645/AI/NIAID NIH HHS/United States
- R03 HD072796/HD/NICHD NIH HHS/United States
- P30 AI042845/AI/NIAID NIH HHS/United States
- U01 AI046725/AI/NIAID NIH HHS/United States
- U01 AI068616/AI/NIAID NIH HHS/United States
- UM1 AI068616/AI/NIAID NIH HHS/United States
- 1R03-HD072796-01A1/HD/NICHD NIH HHS/United States
- U01 AI068632/AI/NIAID NIH HHS/United States
- UM1 AI068632/AI/NIAID NIH HHS/United States
- 5P30 AI064518/AI/NIAID NIH HHS/United States
- 5UO1 AI46725/AI/NIAID NIH HHS/United States
- KL2 TR001115/TR/NCATS NIH HHS/United States
- U01 AI041110/AI/NIAID NIH HHS/United States
- Y1-AI-2642-12/AI/NIAID NIH HHS/United States
- 1 U01 AI068616/AI/NIAID NIH HHS/United States
- 5 U01 AI41110/AI/NIAID NIH HHS/United States
- UM1-AI100645-01/AI/NIAID NIH HHS/United States
- KL2TR001115/TR/NCATS NIH HHS/United States
- P30 AI064518/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

