Prolonged neuroinflammation after lipopolysaccharide exposure in aged rats
- PMID: 25170959
- PMCID: PMC4149545
- DOI: 10.1371/journal.pone.0106331
Prolonged neuroinflammation after lipopolysaccharide exposure in aged rats
Abstract
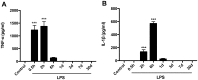
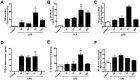
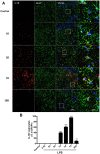
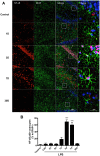
Inflammation is a hallmark of several disease states ranging from neurodegeneration to sepsis but is also implicated in physiological processes like ageing. Non-resolving inflammation and prolonged neuroinflammation are unclear processes implicated in several conditions, including ageing. In this study we studied the long-term effects of endotoxemia, as systemic lipopolysaccharide (LPS) injection, focusing on the role of astrocyte activation and cytokine release in the brain of aged rats. A single dose of LPS (2 mg/kg) or 0.9% saline was injected intraperitoneally in aged rats. Levels of pro-inflammatory cytokines (TNFα and IL-1β) and NF-κB p65 activation were measured systemically and in hippocampal tissue. Astrocytes and cytokines release in the CNS were detected via double immunofluorescence staining at different time-points up to day 30. Serum levels of TNFα and IL-1β were significantly increased acutely after 30 minutes (p<0.001) and up to 6 hours (p<0.001) following LPS-injection. Centrally, LPS-treated rats showed up-regulated mRNA expression and protein levels of pro-inflammatory cytokines in the hippocampus. These changes associated with astrogliosis in the hippocampus dentate gyrus (DG), IL-1β immunoreactivity and elevated NF-κB p65 expression up to day 30 post LPS exposure. Overall, these data demonstrate that LPS induces prolonged neuroinflammation and astrocyte activation in the hippocampus of aged rats. Hippocampal NF-κB p65 and excessive astrocytes-derived IL-1β release may play a pivotal role in regulating long-lasting neuroinflammation.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

