Zebrafish WNK lysine deficient protein kinase 1 (wnk1) affects angiogenesis associated with VEGF signaling
- PMID: 25171174
- PMCID: PMC4149531
- DOI: 10.1371/journal.pone.0106129
Zebrafish WNK lysine deficient protein kinase 1 (wnk1) affects angiogenesis associated with VEGF signaling
Abstract
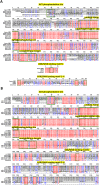
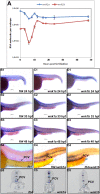
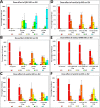
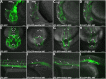
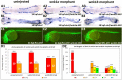
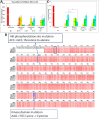
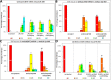
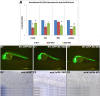
The WNK1 (WNK lysine deficient protein kinase 1) protein is a serine/threonine protein kinase with emerging roles in cancer. WNK1 causes hypertension and hyperkalemia when overexpressed and cardiovascular defects when ablated in mice. In this study, the role of Wnk1 in angiogenesis was explored using the zebrafish model. There are two zebrafish wnk1 isoforms, wnk1a and wnk1b, and both contain all the functional domains found in the human WNK1 protein. Both isoforms are expressed in the embryo at the initiation of angiogenesis and in the posterior cardinal vein (PCV), similar to fms-related tyrosine kinase 4 (flt4). Using morpholino antisense oligonucleotides against wnk1a and wnk1b, we observed that wnk1 morphants have defects in angiogenesis in the head and trunk, similar to flk1/vegfr2 morphants. Furthermore, both wnk1a and wnk1b mRNA can partially rescue the defects in vascular formation caused by flk1/vegfr2 knockdown. Mutation of the kinase domain or the Akt/PI3K phosphorylation site within wnk1 destroys this rescue capability. The rescue experiments provide evidence that wnk1 is a downstream target for Vegfr2 (vascular endothelial growth factor receptor-2) and Akt/PI3K signaling and thereby affects angiogenesis in zebrafish embryos. Furthermore, we found that knockdown of vascular endothelial growth factor receptor-2 (flk1/vegfr2) or vascular endothelial growth factor receptor-3 (flt4/vegfr3) results in a decrease in wnk1a expression, as assessed by in situ hybridization and q-RT-PCR analysis. Thus, the Vegf/Vegfr signaling pathway controls angiogenesis in zebrafish via Akt kinase-mediated phosphorylation and activation of Wnk1 as well as transcriptional regulation of wnk1 expression.
Conflict of interest statement
Figures
References
-
- Baldessari D, Mione M (2008) How to create the vascular tree? (Latest) help from the zebrafish. Pharmacol Ther 118: 206–230. - PubMed
-
- Risau W (1997) Mechanisms of angiogenesis. Nature 386: 671–674. - PubMed
-
- Shibuya M (2008) Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep 41: 278–286. - PubMed
-
- Jiang ZY, Zhou QL, Holik J, Patel S, Leszyk J, et al. (2005) Identification of WNK1 as a substrate of Akt/protein kinase B and a negative regulator of insulin-stimulated mitogenesis in 3T3-L1 cells. J Biol Chem 280: 21622–21628. - PubMed
-
- Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, et al. (2000) WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 275: 16795–16801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

