Dihydropyrimidine accumulation is required for the epithelial-mesenchymal transition
- PMID: 25171410
- PMCID: PMC4250222
- DOI: 10.1016/j.cell.2014.07.032
Dihydropyrimidine accumulation is required for the epithelial-mesenchymal transition
Abstract
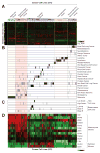
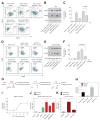
It is increasingly appreciated that oncogenic transformation alters cellular metabolism to facilitate cell proliferation, but less is known about the metabolic changes that promote cancer cell aggressiveness. Here, we analyzed metabolic gene expression in cancer cell lines and found that a set of high-grade carcinoma lines expressing mesenchymal markers share a unique 44 gene signature, designated the "mesenchymal metabolic signature" (MMS). A FACS-based shRNA screen identified several MMS genes as essential for the epithelial-mesenchymal transition (EMT), but not for cell proliferation. Dihydropyrimidine dehydrogenase (DPYD), a pyrimidine-degrading enzyme, was highly expressed upon EMT induction and was necessary for cells to acquire mesenchymal characteristics in vitro and for tumorigenic cells to extravasate into the mouse lung. This role of DPYD was mediated through its catalytic activity and enzymatic products, the dihydropyrimidines. Thus, we identify metabolic processes essential for the EMT, a program associated with the acquisition of metastatic and aggressive cancer cell traits.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




References
-
- Amstutz U, Froehlich TK, Largiadèr CR. Dihydropyrimidine dehydrogenase gene as a major predictor of severe 5-fluorouracil toxicity. Pharmacogenomics. 2011;12:1321–1336. - PubMed
-
- Benesch MGK, Tang X, Maeda T, Ohhata A, Zhao YY, Kok BPC, Dewald J, Hitt M, Curtis JM, McMullen TPW, et al. Inhibition of autotaxin delays breast tumor growth and lung metastasis in mice. Faseb J. 2014;28:2655–2666. - PubMed
-
- Brabletz T. To differentiate or not — routes towards metastasis. Nat Rev Cancer. 2012;12:425–436. - PubMed
-
- Büchel B, Rhyn P, Schürch S, Bühr C, Amstutz U, Largiadèr RC. LC-MS/MS method for simultaneous analysis of uracil, 5,6-dihydrouracil, 5-fluorouracil and 5-fluoro-5,6-dihydrouracil in human plasma for therapeutic drug monitoring and toxicity prediction in cancer patients. Cancer Sci. 2012;27:7–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

