Temporal patterning of neuroblasts controls Notch-mediated cell survival through regulation of Hid or Reaper
- PMID: 25171415
- PMCID: PMC4153738
- DOI: 10.1016/j.cell.2014.07.045
Temporal patterning of neuroblasts controls Notch-mediated cell survival through regulation of Hid or Reaper
Abstract
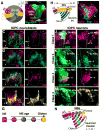
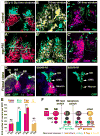
Temporal patterning of neural progenitors is one of the core mechanisms generating neuronal diversity in the central nervous system. Here, we show that, in the tips of the outer proliferation center (tOPC) of the developing Drosophila optic lobes, a unique temporal series of transcription factors not only governs the sequential production of distinct neuronal subtypes but also controls the mode of progenitor division, as well as the selective apoptosis of Notch(OFF) or Notch(ON) neurons during binary cell fate decisions. Within a single lineage, intermediate precursors initially do not divide and generate only one neuron; subsequently, precursors divide, but their Notch(ON) progeny systematically die through Reaper activity, whereas later, their Notch(OFF) progeny die through Hid activity. These mechanisms dictate how the tOPC produces neurons for three different optic ganglia. We conclude that temporal patterning generates neuronal diversity by specifying both the identity and survival/death of each unique neuronal subtype.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





References
-
- Baumgardt M, Karlsson D, Terriente J, Diaz-Benjumea FJ, Thor S. Neuronal subtype specification within a lineage by opposing temporal feed-forward loops. Cell. 2009;139:969–982. - PubMed
-
- Brody T, Odenwald WF. Programmed transformations in neuroblast gene expression during Drosophila CNS lineage development. Developmental biology. 2000;226:34–44. - PubMed
-
- Buescher M, Yeo SL, Udolph G, Zavortink M, Yang X, Tear G, Chia W. Binary sibling neuronal cell fate decisions in the Drosophila embryonic central nervous system are nonstochastic and require inscuteable-mediated asymmetry of ganglion mother cells. Genes & development. 1998;12:1858–1870. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

