Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp
- PMID: 25171469
- PMCID: PMC4214273
- DOI: 10.1038/nature13777
Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp
Abstract
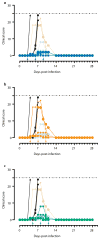
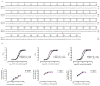
Without an approved vaccine or treatments, Ebola outbreak management has been limited to palliative care and barrier methods to prevent transmission. These approaches, however, have yet to end the 2014 outbreak of Ebola after its prolonged presence in West Africa. Here we show that a combination of monoclonal antibodies (ZMapp), optimized from two previous antibody cocktails, is able to rescue 100% of rhesus macaques when treatment is initiated up to 5 days post-challenge. High fever, viraemia and abnormalities in blood count and blood chemistry were evident in many animals before ZMapp intervention. Advanced disease, as indicated by elevated liver enzymes, mucosal haemorrhages and generalized petechia could be reversed, leading to full recovery. ELISA and neutralizing antibody assays indicate that ZMapp is cross-reactive with the Guinean variant of Ebola. ZMapp exceeds the efficacy of any other therapeutics described so far, and results warrant further development of this cocktail for clinical use.
Figures



Comment in
-
Medical research: Ebola therapy protects severely ill monkeys.Nature. 2014 Oct 2;514(7520):41-3. doi: 10.1038/nature13746. Epub 2014 Aug 29. Nature. 2014. PMID: 25171470 Free PMC article.
-
New hope in the search for Ebola virus treatments.Immunity. 2014 Oct 16;41(4):515-7. doi: 10.1016/j.immuni.2014.10.001. Immunity. 2014. PMID: 25367568
-
How to turn competitors into collaborators.Nature. 2017 Jan 18;541(7637):283-285. doi: 10.1038/541283a. Nature. 2017. PMID: 28102273 No abstract available.
References
-
- WHO.int. WHO - Ebola virus disease (EVD) 2014 < http://www.who.int/csr/don/archive/disease/ebola/en/>.
-
- CDC.gov. Chronology of Ebola Hemorrhagic Fever Outbreaks. 2014 < http://www.cdc.gov/vhf/ebola/resources/outbreak-table.html>.
-
- Reliefweb.int. W. African Ebola epidemic ‘likely to last months’: UN. 2014 < http://reliefweb.int/report/guinea/w-african-ebola-epidemic-likely-last-...>.

