Enhanced innate type 2 immune response in peripheral blood from patients with asthma
- PMID: 25171868
- PMCID: PMC4149890
- DOI: 10.1016/j.jaci.2014.06.024
Enhanced innate type 2 immune response in peripheral blood from patients with asthma
Abstract
Background: In mice, group 2 innate lymphoid cells (ILC2s) likely mediate helminth immunity, inflammation, and tissue repair and remodeling. However, the involvement of ILC2s in human diseases, such as asthma, is not well understood.
Objectives: The goals of this study were to investigate whether peripheral blood specimens can be used to monitor innate type 2 immunity in human subjects and to examine whether ILC2s are involved in human asthma.
Methods: PBMCs from subjects with allergic asthma (AA), subjects with allergic rhinitis (AR), or healthy control (HC) subjects were cultured in vitro with IL-25 or IL-33. Flow cytometry and cell sorting were used to identify, isolate, and quantitate ILC2s in PBMCs.
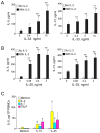
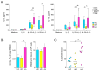
Results: Human PBMCs produced IL-5 and IL-13 when stimulated with IL-33 or IL-25 in the presence of IL-2 without antigens. In addition, IL-7 or thymic stromal lymphopoietin were able to replace IL-2. The cell population with phenotypic ILC2 characteristics, lineage(-)CD127(+)CRTH2(+) cells, responded to IL-33 and produced large quantities of IL-5 and IL-13 but undetectable levels of IL-4. PBMCs from subjects with AA produced significantly larger amounts of IL-5 and IL-13 in response to IL-25 or IL-33 than from subjects with AR or HC. The prevalence of ILC2s in blood was greater in the AA group than in the AR group or the HC group.
Conclusions: Innate type 2 immune responses are increased in asthma but not in AR, suggesting potential differences in the immunopathogenesis of these diseases. Peripheral blood is useful for evaluating innate type 2 immunity in humans.
Keywords: IL-13; IL-25; IL-33; IL-5; ILC2; allergy; asthma; innate immunity.
Copyright © 2014 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures
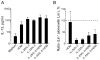

References
-
- From the Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA) 2012 Available from: http://www.ginasthma.org/
-
- Duffy DL, Martin NG, Battistutta D, Hopper JL, Mathews JD. Genetics of asthma and hay fever in Australian twins. Am Rev Respir Dis. 1990;142:1351–8. - PubMed
-
- Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–7. - PubMed
-
- Faul JL, Demers EA, Burke CM, Poulter LW. The reproducibility of repeat measures of airway inflammation in stable atopic asthma. Am J Respir Crit Care Med. 1999;160:1457–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

