The interaction between Shroom3 and Rho-kinase is required for neural tube morphogenesis in mice
- PMID: 25171888
- PMCID: PMC4163662
- DOI: 10.1242/bio.20147450
The interaction between Shroom3 and Rho-kinase is required for neural tube morphogenesis in mice
Abstract
Shroom3 is an actin-associated regulator of cell morphology that is required for neural tube closure, formation of the lens placode, and gut morphogenesis in mice and has been linked to chronic kidney disease and directional heart looping in humans. Numerous studies have shown that Shroom3 likely regulates these developmental processes by directly binding to Rho-kinase and facilitating the assembly of apically positioned contractile actomyosin networks. We have characterized the molecular basis for the neural tube defects caused by an ENU-induced mutation that results in an arginine-to-cysteine amino acid substitution at position 1838 of mouse Shroom3. We show that this substitution has no effect on Shroom3 expression or localization but ablates Rock binding and renders Shroom3 non-functional for the ability to regulate cell morphology. Our results indicate that Rock is the major downstream effector of Shroom3 in the process of neural tube morphogenesis. Based on sequence conservation and biochemical analysis, we predict that the Shroom-Rock interaction is highly conserved across animal evolution and represents a signaling module that is utilized in a variety of biological processes.
Keywords: Rock; Shroom3; apical constriction; epithelial; neural tube.
© 2014. Published by The Company of Biologists Ltd.
Conflict of interest statement
Figures



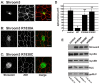
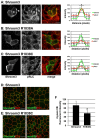
Similar articles
-
Shroom3 functions downstream of planar cell polarity to regulate myosin II distribution and cellular organization during neural tube closure.Biol Open. 2015 Jan 16;4(2):186-96. doi: 10.1242/bio.20149589. Biol Open. 2015. PMID: 25596276 Free PMC article.
-
Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling.Development. 2008 Apr;135(8):1493-502. doi: 10.1242/dev.019646. Epub 2008 Mar 13. Development. 2008. PMID: 18339671
-
A Trio-RhoA-Shroom3 pathway is required for apical constriction and epithelial invagination.Development. 2011 Dec;138(23):5177-88. doi: 10.1242/dev.067868. Epub 2011 Oct 26. Development. 2011. PMID: 22031541 Free PMC article.
-
The Critical Role of the Shroom Family Proteins in Morphogenesis, Organogenesis and Disease.Phenomics. 2024 Mar 6;4(2):187-202. doi: 10.1007/s43657-023-00119-9. eCollection 2024 Apr. Phenomics. 2024. PMID: 38884059 Free PMC article. Review.
-
The Good and the Bad of SHROOM3 in Kidney Development and Disease: A Narrative Review.Can J Kidney Health Dis. 2023 Dec 13;10:20543581231212038. doi: 10.1177/20543581231212038. eCollection 2023. Can J Kidney Health Dis. 2023. PMID: 38107159 Free PMC article. Review.
Cited by
-
Genetic and functional analysis of SHROOM1-4 in a Chinese neural tube defect cohort.Hum Genet. 2018 Mar;137(3):195-202. doi: 10.1007/s00439-017-1864-x. Epub 2018 Feb 8. Hum Genet. 2018. PMID: 29423651 Free PMC article.
-
The RhoGEF protein Plekhg5 regulates medioapical and junctional actomyosin dynamics of apical constriction during Xenopus gastrulation.Mol Biol Cell. 2023 Jun 1;34(7):ar64. doi: 10.1091/mbc.E22-09-0411. Epub 2023 Apr 12. Mol Biol Cell. 2023. PMID: 37043306 Free PMC article.
-
Folic acid modifies the shape of epithelial cells during morphogenesis via a Folr1 and MLCK dependent mechanism.Biol Open. 2019 Jan 22;8(1):bio041160. doi: 10.1242/bio.041160. Biol Open. 2019. PMID: 30670450 Free PMC article.
-
Assays for Apical Constriction Using the Xenopus Model.Methods Mol Biol. 2022;2438:415-437. doi: 10.1007/978-1-0716-2035-9_24. Methods Mol Biol. 2022. PMID: 35147955 Free PMC article.
-
From morphogen to morphogenesis and back.Nature. 2017 Jan 18;541(7637):311-320. doi: 10.1038/nature21348. Nature. 2017. PMID: 28102269 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

