Synthesis of α-L-rhamnosyl ceramide and evaluation of its binding with anti-rhamnose antibodies
- PMID: 25172148
- PMCID: PMC4172545
- DOI: 10.1016/j.bmc.2014.08.002
Synthesis of α-L-rhamnosyl ceramide and evaluation of its binding with anti-rhamnose antibodies
Abstract
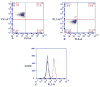
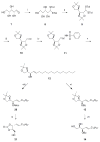
An α-L-rhamnosyl ceramide (1, α-L-RhaCer) has been prepared that was recognized by anti-L-rhamnose (anti-Rha) antibodies. During these studies we explored the use of an α-L-rhamnosyl thioglycoside and a trichloroacetimidate as a glycosyl donors. Subsequently, the acceptors desired for glycosylation, 3-O-benzoylazidosphingosine or 3-O-alloxycarbonylsphingosine, were prepared from D-xylose. The thioglycoside donor, 2,3,4-tri-O-acetyl-1-(4-tolyl)thio-α-L-rhamnopyranoside, and the trichloroacetimidate donor, 2,3,4-tri-O-acetyl-1-(2,2,2-trichloroethanimidate)-α-L-rhamnopyranoside, were synthesized in 50% and 78% yield overall, respectively. The synthesis of the glycosylation acceptor employed an addition-fragmentation olefination that was successfully carried out in 53% yield. With the successful synthesis of key intermediates, α-L-RhaCer (1) was prepared without any insurmountable obstacles. Anti-Rha antibodies were prepared in BALB/c mice by immunizing them with rhamnose-ovalbumin (Rha-Ova) with Sigma Adjuvant System (SAS) and the anti-L-Rha antibodies were isolated from the blood sera. Liposomes and EL4 tumor cells were used as model systems to demonstrate the ability of 1 to insert into a lipid bilayer. The interaction of the liposomes or the EL4 cells with α-L-RhaCer (1) and anti-Rha antibodies were investigated by fluorescence microscopy and flow cytometry, respectively, to confirm the ability of glycolipid 1 to be displayed on the tumor cell surface as well as the ability to be recognized by anti-Rha antibodies.
Keywords: Anti-cancer vaccine; Anti-rhamnose antibody; Glycolipid; Rhamnosyl-ceramide.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures







Similar articles
-
Synthesis of a single-molecule L-rhamnose-containing three-component vaccine and evaluation of antigenicity in the presence of anti-L-rhamnose antibodies.J Am Chem Soc. 2010 Dec 8;132(48):17236-46. doi: 10.1021/ja107029z. Epub 2010 Nov 16. J Am Chem Soc. 2010. PMID: 21080675
-
Synthesis and immunological evaluation of a MUC1 glycopeptide incorporated into l-rhamnose displaying liposomes.Bioconjug Chem. 2013 Mar 20;24(3):363-75. doi: 10.1021/bc300422a. Epub 2013 Mar 8. Bioconjug Chem. 2013. PMID: 23444835 Free PMC article.
-
Augmenting Vaccine Immunogenicity through the Use of Natural Human Anti-rhamnose Antibodies.ACS Chem Biol. 2018 Aug 17;13(8):2130-2142. doi: 10.1021/acschembio.8b00312. Epub 2018 Jul 2. ACS Chem Biol. 2018. PMID: 29916701 Free PMC article.
-
Recent studies on reaction pathways and applications of sugar orthoesters in synthesis of oligosaccharides.Carbohydr Res. 2007 Feb 26;342(3-4):345-73. doi: 10.1016/j.carres.2006.09.025. Epub 2006 Oct 5. Carbohydr Res. 2007. PMID: 17109835 Review.
-
Synthesis of the beta-rhamnopyranosides and the 6-deoxy-beta-mannoheptopyranosides.Chimia (Aarau). 2011;65(1-2):59-64. doi: 10.2533/chimia.2011.59. Chimia (Aarau). 2011. PMID: 21469447 Review.
Cited by
-
Synthesis of a Liposomal MUC1 Glycopeptide-Based Immunotherapeutic and Evaluation of the Effect of l-Rhamnose Targeting on Cellular Immune Responses.Bioconjug Chem. 2016 Jan 20;27(1):110-20. doi: 10.1021/acs.bioconjchem.5b00528. Epub 2015 Dec 9. Bioconjug Chem. 2016. PMID: 26595674 Free PMC article.
-
Design, Synthesis, and Immunological Evaluation of Benzyloxyalkyl-Substituted 1,2,3-Triazolyl α-GalCer Analogues.ACS Med Chem Lett. 2015 Dec 10;7(2):172-6. doi: 10.1021/acsmedchemlett.5b00340. eCollection 2016 Feb 11. ACS Med Chem Lett. 2015. PMID: 26985293 Free PMC article.
-
Synthesis of Chrysogeside B from Halotolerant Fungus Penicillium and Its Antimicrobial Activities Evaluation.Sci Rep. 2017 Apr 11;7:45927. doi: 10.1038/srep45927. Sci Rep. 2017. PMID: 28397807 Free PMC article.
-
Monophosphoryl Lipid A-Rhamnose Conjugates as a New Class of Vaccine Adjuvants.J Med Chem. 2024 May 9;67(9):7458-7469. doi: 10.1021/acs.jmedchem.3c02385. Epub 2024 Apr 18. J Med Chem. 2024. PMID: 38634150 Free PMC article.
-
Highly efficient chemoenzymatic synthesis and facile purification of α-Gal pentasaccharyl ceramide Galα3nLc4βCer.Chem Commun (Camb). 2017 Jul 20;53(59):8280-8283. doi: 10.1039/c7cc04090c. Chem Commun (Camb). 2017. PMID: 28695219 Free PMC article.
References
-
- Oyelaran O, McShane ML, Dodd L, Gildersleeve JC. J Proteome Res. 2009;8:4301. - PMC - PubMed
- Huflejt ME, Vuskovic M, Vasiliu D, Xu H, Obukhova P, Shilova N, Tuzikov A, Galanina O, Arun B, Lu K, Bovin N. Mol Immunol. 2009;46:3037. - PubMed
- Schwarz M, Spector L, Gargir A, Shtevi A, Gortler M, Alstock RT, Dukler AA, Dotan N. Glycobiology. 2003;13:749. - PubMed
- Sheridan RTC, Hudon J, Hank JA, Sondel PM, Kiessling LL. ChemBioChem. 2014 doi: 10.1002/cbic.201402019. - DOI - PMC - PubMed
-
- Shokat KM, Schultz PG. J Am Chem Soc. 1991;113:1861.
- Bertozzi CR, Bednarski MD. J Am Chem Soc. 1992;114:5543.
- Lussow AR, Buelow R, Fanget L, Peretto S, Gao L, Pouletty P. J Immunother. 1996;19:257. - PubMed
- Li J, Zacharek S, Chen X, Wang J, Zhang W, Janczuk A, Wang PG. Bioorg Med Chem. 1999;7:1549. - PubMed
- Carlson CB, Mowery P, Owen RM, Dykhuizen EC, Kiessling LL. ACS Chem Biol. 2007;2:119. - PubMed
- Parker CG, Domaoal RA, Anderson KS, Spiegel DA. J Am Chem Soc. 2009;131:16392. - PMC - PubMed
-
- Mizutani Y, Kihara A, Chiba H, Hiromasa T, Igarashi Y. J Lipid Res. 2008;49:2356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

