CD39 and CD161 modulate Th17 responses in Crohn's disease
- PMID: 25172498
- PMCID: PMC4170017
- DOI: 10.4049/jimmunol.1400346
CD39 and CD161 modulate Th17 responses in Crohn's disease
Abstract
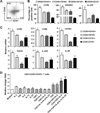
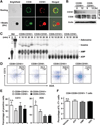
CD39 (ENTPD1) is expressed by subsets of pathogenic human CD4(+) T cells, such as Th17 cells. These Th17 cells are considered important in intestinal inflammation, such as seen in Crohn's disease (CD). Recently, CD161 (NKR-P1A) was shown to be a phenotypic marker of human Th17 cells. In this study, we report that coexpression of CD161 and CD39 not only identifies these cells but also promotes Th17 generation. We note that human CD4(+)CD39(+)CD161(+) T cells can be induced under stimulatory conditions that promote Th17 in vitro. Furthermore, CD4(+)CD39(+)CD161(+) cells purified from blood and intestinal tissues, from both healthy controls and patients with CD, are of the Th17 phenotype and exhibit proinflammatory functions. CD39 is coexpressed with CD161, and this association augments acid sphingomyelinase (ASM) activity upon stimulation of CD4(+) T cells. These pathways regulate mammalian target of rapamycin and STAT3 signaling to drive the Th17 phenotype. Inhibition of ASM activity by pharmacological blockers or knockdown of ASM abrogates STAT3 signaling, thereby limiting IL-17 production in CD4(+) T cells obtained from both controls and patients with active CD. Increased levels of CD39(+)CD161(+) CD4(+) T cells in blood or lamina propria are noted in patients with CD, and levels directly correlate with clinical disease activity. Hence, coexpression of CD39 and CD161 by CD4(+) T cells might serve as a biomarker to monitor Th17 responsiveness. Collectively, CD39 and CD161 modulate human Th17 responses in CD through alterations in purinergic nucleotide-mediated responses and ASM catalytic bioactivity, respectively.
Copyright © 2014 by The American Association of Immunologists, Inc.
Conflict of interest statement
Figures





References
-
- Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. - PubMed
-
- Brand S. Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut. 2009;58:1152–1167. - PubMed
-
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5T32DK007760-15/DK/NIDDK NIH HHS/United States
- R01 HL062458/HL/NHLBI NIH HHS/United States
- R21 CA164970/CA/NCI NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- P01 AI045897/AI/NIAID NIH HHS/United States
- DP1 CA174427/CA/NCI NIH HHS/United States
- R01 HL094400/HL/NHLBI NIH HHS/United States
- R01-HL094400/HL/NHLBI NIH HHS/United States
- G0902288/MRC_/Medical Research Council/United Kingdom
- T32 DK007760/DK/NIDDK NIH HHS/United States
- K23 DK084338/DK/NIDDK NIH HHS/United States
- MR/J006742/1/MRC_/Medical Research Council/United Kingdom
- P01-HL076540/HL/NHLBI NIH HHS/United States
- P01 HL076540/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

