Biosynthetic pathway for the cyanide-free production of phenylacetonitrile in Escherichia coli by utilizing plant cytochrome P450 79A2 and bacterial aldoxime dehydratase
- PMID: 25172862
- PMCID: PMC4249030
- DOI: 10.1128/AEM.01623-14
Biosynthetic pathway for the cyanide-free production of phenylacetonitrile in Escherichia coli by utilizing plant cytochrome P450 79A2 and bacterial aldoxime dehydratase
Abstract
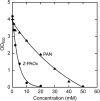
The biosynthetic pathway for the production of phenylacetonitrile (PAN), which has a wide variety of uses in chemical and pharmaceutical industries, was constructed in Escherichia coli utilizing enzymes from the plant glucosinolate-biosynthetic and bacterial aldoxime-nitrile pathways. First, the single-step reaction to produce E,Z-phenylacetaldoxime (PAOx) from l-Phe was constructed in E. coli by introducing the genes encoding cytochrome P450 (CYP) 79A2 and CYP reductase from Arabidopsis thaliana, yielding the E,Z-PAOx-producing transformant. Second, this step was expanded to the production of PAN by further introducing the aldoxime dehydratase (Oxd) gene from Bacillus sp. strain OxB-1, yielding the PAN-producing transformant. The E,Z-PAOx-producing transformant also produced phenethyl alcohol and PAN as by-products, which were suggested to be the metabolites of E,Z-PAOx produced by E. coli enzymes, while the PAN-producing transformant accumulated only PAN in the culture broth, which suggested that the CYP79A2 reaction (the conversion of l-Phe to E,Z-PAOx) was a potential bottleneck in the PAN production pathway. Expression of active CYP79A2 and concentration of biomass were improved by the combination of the autoinduction method, coexpression of groE, encoding the heat shock protein GroEL/GroES, N-terminal truncation of CYP79A2, and optimization of the culture conditions, yielding a >60-fold concentration of E,Z-PAOx (up to 2.9 mM). The concentration of PAN was 4.9 mM under the optimized conditions. These achievements show the potential of this bioprocess to produce nitriles and nitrile derivatives in the absence of toxic chemicals.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Cytochrome P450 CYP71AT96 catalyses the final step of herbivore-induced phenylacetonitrile biosynthesis in the giant knotweed, Fallopia sachalinensis.Plant Mol Biol. 2016 Jun;91(3):229-39. doi: 10.1007/s11103-016-0459-6. Epub 2016 Feb 29. Plant Mol Biol. 2016. PMID: 26928800
-
Identification and characterization of cytochrome P450 CYP77A59 of loquat (Rhaphiolepis bibas) responsible for biosynthesis of phenylacetonitrile, a floral nitrile compound.Planta. 2023 May 11;257(6):114. doi: 10.1007/s00425-023-04151-x. Planta. 2023. PMID: 37166515
-
Cytochrome P450 CYP79A2 from Arabidopsis thaliana L. Catalyzes the conversion of L-phenylalanine to phenylacetaldoxime in the biosynthesis of benzylglucosinolate.J Biol Chem. 2000 May 12;275(19):14659-66. doi: 10.1074/jbc.275.19.14659. J Biol Chem. 2000. PMID: 10799553
-
Exploration and utilization of novel aldoxime, nitrile, and nitro compounds metabolizing enzymes from plants and arthropods.Biosci Biotechnol Biochem. 2024 Jan 24;88(2):138-146. doi: 10.1093/bbb/zbad168. Biosci Biotechnol Biochem. 2024. PMID: 38017623 Review.
-
Advances and prospects in metabolic engineering of Escherichia coli for L-tryptophan production.World J Microbiol Biotechnol. 2022 Jan 6;38(2):22. doi: 10.1007/s11274-021-03212-1. World J Microbiol Biotechnol. 2022. PMID: 34989926 Review.
Cited by
-
Systematic engineering pinpoints a versatile strategy for the expression of functional cytochrome P450 enzymes in Escherichia coli cell factories.Microb Cell Fact. 2023 Oct 25;22(1):219. doi: 10.1186/s12934-023-02219-7. Microb Cell Fact. 2023. PMID: 37880718 Free PMC article.
-
The genetic and functional analysis of flavor in commercial tomato: the FLORAL4 gene underlies a QTL for floral aroma volatiles in tomato fruit.Plant J. 2020 Aug;103(3):1189-1204. doi: 10.1111/tpj.14795. Epub 2020 Jun 21. Plant J. 2020. PMID: 32369642 Free PMC article.
-
Complete Genome Sequence of an Aldoxime Degrader, Bacillus sp. OxB-1.Genome Announc. 2015 Feb 26;3(1):e00025-15. doi: 10.1128/genomeA.00025-15. Genome Announc. 2015. PMID: 25720679 Free PMC article.
-
Metabolomic Profiling, In Vitro Antimalarial Investigation and In Silico Modeling of the Marine Actinobacterium Strain Rhodococcus sp. UR111 Associated with the Soft Coral Nephthea sp.Antibiotics (Basel). 2022 Nov 15;11(11):1631. doi: 10.3390/antibiotics11111631. Antibiotics (Basel). 2022. PMID: 36421275 Free PMC article.
-
Total biosynthesis of opiates by stepwise fermentation using engineered Escherichia coli.Nat Commun. 2016 Feb 5;7:10390. doi: 10.1038/ncomms10390. Nat Commun. 2016. PMID: 26847395 Free PMC article.
References
-
- Pollak P, Romeder G, Hagedorn F, Gelbke H-P. 2000. Nitriles, p 363–376 In Elvers B, Hawkins S, Schulz G. (ed), Ullmann's encyclopedia of industrial chemistry. Wiley-VCH Verlag, Weinheim, Germany.
-
- Adams RT, Thal AF. 1922. Benzyl cyanide. Org. Synth. Coll. 2:9. 10.15227/orgsyn.002.0009. - DOI
-
- Botteghi C, Chelucci G, Marchetti M. 1982. Synthesis of optically active aliphatic nitriles from aldehydes and acids. Synth. Commun. 12:25–33. 10.1080/00397918208080062. - DOI
-
- Fenselau AH, Hamamura EH, Moffatt JG. 1970. Carbodiimide-sulfoxide reactions. VIII. Reactions of oximes and hydroxylamines. J. Org. Chem. 35:3546–3552.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

