Aedes aegypti salivary protein "aegyptin" co-inoculation modulates dengue virus infection in the vertebrate host
- PMID: 25173089
- PMCID: PMC4254699
- DOI: 10.1016/j.virol.2014.07.019
Aedes aegypti salivary protein "aegyptin" co-inoculation modulates dengue virus infection in the vertebrate host
Abstract
Dengue virus (DENV) is transmitted in the saliva of the mosquito vector Aedes aegypti during blood meal acquisition. This saliva is composed of numerous proteins with the capacity to disrupt hemostasis or modulate the vertebrate immune response. One such protein, termed "aegyptin," is an allergen and inhibitor of clot formation, and has been found in decreased abundance in the saliva of DENV-infected mosquitoes. To examine the influence of aegyptin on DENV infection of the vertebrate, we inoculated IRF-3/7(-/- -/-) mice with DENV serotype 2 strain 1232 with and without co-inoculation of aegyptin. Mice that received aegyptin exhibited decreased DENV titers in inoculation sites and in circulation, as well as increased concentrations of GM-CSF, IFN-γ, IL-5, and IL-6, at 48 h post-inoculation when compared to mice that received inoculation of DENV alone. These and other data suggest that aegyptin impacts DENV perpetuation via elevated induction of the immune response.
Keywords: Aegyptin; Allergen; Dengue; Mosquito; Mouse; Saliva; Virus.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


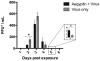
Similar articles
-
Collagen-binding protein, Aegyptin, regulates probing time and blood feeding success in the dengue vector mosquito, Aedes aegypti.Proc Natl Acad Sci U S A. 2014 May 13;111(19):6946-51. doi: 10.1073/pnas.1404179111. Epub 2014 Apr 28. Proc Natl Acad Sci U S A. 2014. PMID: 24778255 Free PMC article.
-
A salivary protein of Aedes aegypti promotes dengue-2 virus replication and transmission.Insect Biochem Mol Biol. 2019 Aug;111:103181. doi: 10.1016/j.ibmb.2019.103181. Epub 2019 Jun 29. Insect Biochem Mol Biol. 2019. PMID: 31265906
-
The roles of IRF-3 and IRF-7 in innate antiviral immunity against dengue virus.J Immunol. 2013 Oct 15;191(8):4194-201. doi: 10.4049/jimmunol.1300799. Epub 2013 Sep 16. J Immunol. 2013. PMID: 24043884 Free PMC article.
-
Analysis of early dengue virus infection in mice as modulated by Aedes aegypti probing.J Virol. 2014 Feb;88(4):1881-9. doi: 10.1128/JVI.01218-13. Epub 2013 Nov 6. J Virol. 2014. PMID: 24198426 Free PMC article.
-
IL-18: The Forgotten Cytokine in Dengue Immunopathogenesis.J Immunol Res. 2021 Nov 19;2021:8214656. doi: 10.1155/2021/8214656. eCollection 2021. J Immunol Res. 2021. PMID: 34840991 Free PMC article. Review.
Cited by
-
Vertebrate Responses against Arthropod Salivary Proteins and Their Therapeutic Potential.Vaccines (Basel). 2021 Apr 5;9(4):347. doi: 10.3390/vaccines9040347. Vaccines (Basel). 2021. PMID: 33916367 Free PMC article. Review.
-
Arbovirus-Mosquito Vector-Host Interactions and the Impact on Transmission and Disease Pathogenesis of Arboviruses.Front Microbiol. 2019 Jan 23;10:22. doi: 10.3389/fmicb.2019.00022. eCollection 2019. Front Microbiol. 2019. PMID: 30728812 Free PMC article. Review.
-
Infecting mosquitoes alters DENV-2 characteristics and enhances hemorrhage-induction potential in Stat1-/- mice.PLoS Negl Trop Dis. 2021 Aug 27;15(8):e0009728. doi: 10.1371/journal.pntd.0009728. eCollection 2021 Aug. PLoS Negl Trop Dis. 2021. PMID: 34449772 Free PMC article.
-
The Vector - Host - Pathogen Interface: The Next Frontier in the Battle Against Mosquito-Borne Viral Diseases?Front Cell Infect Microbiol. 2020 Oct 16;10:564518. doi: 10.3389/fcimb.2020.564518. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33178624 Free PMC article. Review.
-
Safety and immunogenicity of a mosquito saliva peptide-based vaccine: a randomised, placebo-controlled, double-blind, phase 1 trial.Lancet. 2020 Jun 27;395(10242):1998-2007. doi: 10.1016/S0140-6736(20)31048-5. Epub 2020 Jun 11. Lancet. 2020. PMID: 32534628 Free PMC article. Clinical Trial.
References
-
- Crompton DWT, editor. WHO. Working to overcome the global impact of neglected tropical diseases. WHO Press; Geneva, Switzerland: 2010.
-
- Griffiths RB, Gordon RM. An apparatus which enables the process of feeding by mosquitoes to be observed in the tissues of a live rodent; together with an account of the ejection of saliva and its significance in Malaria. Ann Trop Med Parasitol. 1952;46(4):311–9. - PubMed
-
- Turell MJ, Spielman A. Nonvascular delivery of Rift Valley fever virus by infected mosquitoes. Am J Trop Med Hyg. 1992;47(2):190–4. - PubMed
-
- Turell MJ, Tammariello RF, Spielman A. Nonvascular delivery of St. Louis encephalitis and Venezuelan equine encephalitis viruses by infected mosquitoes (Diptera: Culicidae) feeding on a vertebrate host. J Med Entomol. 1995;32(4):563–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

