Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion
- PMID: 25173346
- PMCID: PMC4479254
- DOI: 10.1038/ni.2984
Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion
Abstract
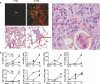
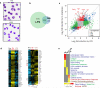
We examined the role of innate cells in acquired resistance to the natural murine parasitic nematode, Nippostrongylus brasiliensis. Macrophages obtained from lungs as late as 45 d after N. brasiliensis inoculation were able to transfer accelerated parasite clearance to naive recipients. Primed macrophages adhered to larvae in vitro and triggered increased mortality of parasites. Neutrophil depletion in primed mice abrogated the protective effects of transferred macrophages and inhibited their in vitro binding to larvae. Neutrophils in parasite-infected mice showed a distinct transcriptional profile and promoted alternatively activated M2 macrophage polarization through secretory factors including IL-13. Differentially activated neutrophils in the context of a type 2 immune response therefore prime a long-lived effector macrophage phenotype that directly mediates rapid nematode damage and clearance.
Figures


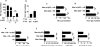


Comment in
-
Neutrophils worm their way into macrophage long-term memory.Nat Immunol. 2014 Oct;15(10):902-4. doi: 10.1038/ni.2990. Nat Immunol. 2014. PMID: 25232812 No abstract available.
References
-
- King CH. Health metrics for helminthic infections. Advances in parasitology. 2010;73:51–69. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

