Comparison of anorectic and emetic potencies of deoxynivalenol (vomitoxin) to the plant metabolite deoxynivalenol-3-glucoside and synthetic deoxynivalenol derivatives EN139528 and EN139544
- PMID: 25173790
- PMCID: PMC4334811
- DOI: 10.1093/toxsci/kfu166
Comparison of anorectic and emetic potencies of deoxynivalenol (vomitoxin) to the plant metabolite deoxynivalenol-3-glucoside and synthetic deoxynivalenol derivatives EN139528 and EN139544
Abstract
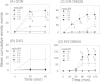
The mycotoxin deoxynivalenol (DON) elicits robust anorectic and emetic effects in several animal species. However, less is known about the potential for naturally occurring and synthetic congeners of this trichothecene to cause analogous responses. Here we tested the hypothesis that alterations in DON structure found in the plant metabolite deoxynivalenol-3-glucoside (D3G) and two pharmacologically active synthetic DON derivatives, EN139528 and EN139544, differentially impact their potential to evoke food refusal and emesis. In a nocturnal mouse food consumption model, oral administration with DON, D3G, EN139528, or EN139544 at doses from 2.5 to 10 mg/kg BW induced anorectic responses that lasted up to 16, 6, 6, and 3 h, respectively. Anorectic potency rank orders were EN139544>DON>EN139528>D3G from 0 to 0.5 h but DON>D3G>EN139528>EN139544 from 0 to 3 h. Oral exposure to each of the four compounds at a common dose (2.5 mg/kg BW) stimulated plasma elevations of the gut satiety peptides cholecystokinin and to a lesser extent, peptide YY3-36 that corresponded to reduced food consumption. In a mink emesis model, oral administration of increasing doses of the congeners differentially induced emesis, causing marked decreases in latency to emesis with corresponding increases in both the duration and number of emetic events. The minimum emetic doses for DON, EN139528, D3G, and EN139544 were 0.05, 0.5, 2, and 5 mg/kg BW, respectively. Taken together, the results suggest that although all three DON congeners elicited anorectic responses that mimicked DON over a narrow dose range, they were markedly less potent than the parent mycotoxin at inducing emesis.
Keywords: 5-hydroxytryptamine; EN139528; EN139544; anorexia; cholecystokinin; deoxynivalenol; deoxynivalenol-3-glucoside; emesis; mycotoxin; peptide YY3–36.
© The Author 2014. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures












References
-
- Amuzie C. J., Harkema J. R., Pestka J. J. Tissue distribution and proinflammatory cytokine induction by the trichothecene deoxynivalenol in the mouse: Comparison of nasal vs. oral exposure. Toxicology. 2008;248:39–44. - PubMed
-
- Azcona-Olivera J. I., Ouyang Y., Murtha J., Chu F. S., Pestka J. J. Induction of cytokine mRNAs in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): Relationship to toxin distribution and protein synthesis inhibition. Toxicol. Appl. Pharmacol. 1995;133:109–120. - PubMed
-
- Batterham R. L., Cowley M. A., Small C. J., Herzog H., Cohen M. A., Dakin C. L., Wren A. M., Brynes A. E., Low M. J., Ghatei M. A. Gut hormone PYY3–36 physiologically inhibits food intake. Nature. 2002;418:650–654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

