Diverse functions of kindlin/fermitin proteins during embryonic development in Xenopus laevis
- PMID: 25173804
- PMCID: PMC4195491
- DOI: 10.1016/j.mod.2014.07.004
Diverse functions of kindlin/fermitin proteins during embryonic development in Xenopus laevis
Abstract
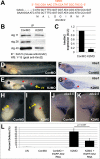
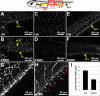
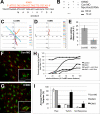
The kindlin/fermitin family includes three proteins involved in regulating integrin ligand-binding activity and adhesion. Loss-of-function mutations in kindlins1 and 3 have been implicated in Kindler Syndrome and Leukocyte Adhesion Deficiency III (LAD-III) respectively, whereas kindlin2 null mice are embryonic lethal. Post translational regulation of cell-cell and cell-ECM adhesion has long been presumed to be important for morphogenesis, however, few specific examples of activation-dependent changes in adhesion molecule function in normal development have been reported. In this study, antisense morpholinos were used to reduce expression of individual kindlins in Xenopus laevis embryos in order to investigate their roles in early development. Kindlin1 knockdown resulted in developmental delays, gross malformations of the gut and eventual lethality by tadpole stages. Kindlin2 morphant embryos displayed late stage defects in vascular maintenance and angiogenic branching consistent with kindlin2 loss of function in the mouse. Antisense morpholinos were also used to deplete maternal kindlin2 protein in oocytes and eggs. Embryos lacking maternal kindlin2 arrested at early cleavage stages due to failures in cytokinesis. Kindlin3 morphant phenotypes included defects in epidermal ciliary beating and partial paralysis at tailbud stages but these embryos recovered eventually as morpholino levels decayed. These results indicate a remarkably diverse range of kindlin functions in vertebrate development.
Keywords: Cardiovascular development; Cilia; Fermitin; Integrin activation; Kindlin.
Copyright © 2014 Elsevier Ireland Ltd. All rights reserved.
Figures





Similar articles
-
Developmental expression of the fermitin/kindlin gene family in Xenopus laevis embryos.Dev Dyn. 2011 Aug;240(8):1958-63. doi: 10.1002/dvdy.22683. Dev Dyn. 2011. PMID: 21761481
-
Kindlin2 regulates neural crest specification via integrin-independent regulation of the FGF signaling pathway.Development. 2021 May 15;148(10):dev199441. doi: 10.1242/dev.199441. Epub 2021 May 17. Development. 2021. PMID: 33999995
-
Use of fully modified 2'-O-methyl antisense oligos for loss-of-function studies in vertebrate embryos.Genesis. 2011 Mar;49(3):117-23. doi: 10.1002/dvg.20689. Genesis. 2011. PMID: 21442720 Free PMC article.
-
Morpholinos Do Not Elicit an Innate Immune Response during Early Xenopus Embryogenesis.Dev Cell. 2019 May 20;49(4):643-650.e3. doi: 10.1016/j.devcel.2019.04.019. Dev Cell. 2019. PMID: 31112700 Free PMC article. Review.
-
The kindlin family: functions, signaling properties and implications for human disease.J Cell Sci. 2016 Jan 1;129(1):17-27. doi: 10.1242/jcs.161190. J Cell Sci. 2016. PMID: 26729028 Review.
Cited by
-
Kindlin-2 directly binds actin and regulates integrin outside-in signaling.J Cell Biol. 2016 Apr 11;213(1):97-108. doi: 10.1083/jcb.201501006. Epub 2016 Apr 4. J Cell Biol. 2016. PMID: 27044892 Free PMC article.
-
Kindlin-2 in myoepithelium controls luminal progenitor commitment to alveoli in mouse mammary gland.Cell Death Dis. 2023 Oct 13;14(10):675. doi: 10.1038/s41419-023-06184-2. Cell Death Dis. 2023. PMID: 37833248 Free PMC article.
-
[Kindlin-2 regulates endometrium development via mTOR and Hippo signaling pathways in mice].Beijing Da Xue Xue Bao Yi Xue Ban. 2022 Oct 18;54(5):846-852. doi: 10.19723/j.issn.1671-167X.2022.05.012. Beijing Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 36241227 Free PMC article. Chinese.
References
-
- Bussolino F, Caccavari F, Valdembri D, Serini G. Angiogenesis: a balancing act between integrin activation and inhibition? Eur Cytokine Netw. 2009;20:191–6. - PubMed
-
- Byzova TV, Goldman CK, Pampori N, Thomas KA, Bett A, Shattil SJ, Plow EF. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell. 2000;6:851–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

