Biomechanical forces in the skeleton and their relevance to bone metastasis: biology and engineering considerations
- PMID: 25174311
- PMCID: PMC4258455
- DOI: 10.1016/j.addr.2014.08.009
Biomechanical forces in the skeleton and their relevance to bone metastasis: biology and engineering considerations
Abstract
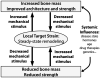
Bone metastasis represents the leading cause of breast cancer related-deaths. However, the effect of skeleton-associated biomechanical signals on the initiation, progression, and therapy response of breast cancer bone metastasis is largely unknown. This review seeks to highlight possible functional connections between skeletal mechanical signals and breast cancer bone metastasis and their contribution to clinical outcome. It provides an introduction to the physical and biological signals underlying bone functional adaptation and discusses the modulatory roles of mechanical loading and breast cancer metastasis in this process. Following a definition of biophysical design criteria, in vitro and in vivo approaches from the fields of bone biomechanics and tissue engineering that may be suitable to investigate breast cancer bone metastasis as a function of varied mechano-signaling will be reviewed. Finally, an outlook of future opportunities and challenges associated with this newly emerging field will be provided.
Keywords: Biomechanics; Bone metastasis; Breast cancer; Mechanical loading; Tissue engineering.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures







References
-
- Kozlow W, Guise TA. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10:169–180. - PubMed
-
- Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L, Chirgwin JM. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12:6213s–6216s. - PubMed
-
- Guise TA. The vicious cycle of bone metastases. J Musculoskelet Neuronal Interact. 2002;2:570–572. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

