Transforming growth factor β regulates β-catenin expression in lung fibroblast through NF-κB dependent pathway
- PMID: 25175023
- PMCID: PMC4199410
- DOI: 10.3892/ijmm.2014.1916
Transforming growth factor β regulates β-catenin expression in lung fibroblast through NF-κB dependent pathway
Abstract
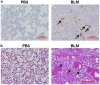
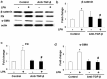
β-catenin contributes to the pathogenesis of lung fibrosis. However, the expression of β-catenin in fibroblasts under fibrotic conditions has not been studied. We investigated the expression of β-catenin in lung fibroblasts from bleomycin (BLM)‑challenged mice and human lung fibroblasts treated with transforming growth factor β (TGF-β) or lysophosphatidic acid (LPA) by western blot analysis. The result showed that the expression of β-catenin was significantly increased in lung fibrotic foci and lung fibroblasts from bleomycin‑challenged mice. TGF-β stimulated β-catenin expression and induced differentiation in human lung fibroblasts in vitro. Pretreatment of the NF-κB activation inhibitor attenuated the TGF-β‑induced expression of β-catenin and differentiation in human lung fibroblasts. Similarly, LPA induced β-catenin expression in human lung fibroblasts, and pre-treatment of the neutralized anti-TGF-β antibody attenuated the LPA‑induced expression of β-catenin and differentiation in human lung fibroblasts. The results suggested that β-catenin expression is upregulated in lung fibroblast during differentiation, and that TGF-β induced β-catenin expression in human lung fibroblasts through the activation of NF-κB.
Figures



Similar articles
-
Transforming growth factor-Beta inhibits heme oxygenase-1 expression in lung fibroblast through nuclear factor-kappa-B-dependent pathway.Pharmacology. 2014;93(3-4):185-92. doi: 10.1159/000360638. Epub 2014 May 20. Pharmacology. 2014. PMID: 24854244
-
P120-catenin regulates pulmonary fibrosis and TGF-β induced lung fibroblast differentiation.Life Sci. 2019 Aug 1;230:35-44. doi: 10.1016/j.lfs.2019.05.052. Epub 2019 May 21. Life Sci. 2019. PMID: 31125560
-
Nuclear factor (NF)-κB p65 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-β.Life Sci. 2015 Feb 1;122:8-14. doi: 10.1016/j.lfs.2014.11.033. Epub 2014 Dec 11. Life Sci. 2015. PMID: 25498897
-
Transforming growth factor-β inhibits IQ motif containing guanosine triphosphatase activating protein 1 expression in lung fibroblasts via the nuclear factor-κB signaling pathway.Mol Med Rep. 2015 Jul;12(1):442-8. doi: 10.3892/mmr.2015.3353. Epub 2015 Feb 13. Mol Med Rep. 2015. PMID: 25684348
-
Sphingolipids in pulmonary fibrosis.Adv Biol Regul. 2015 Jan;57:55-63. doi: 10.1016/j.jbior.2014.09.008. Epub 2014 Oct 13. Adv Biol Regul. 2015. PMID: 25446881 Free PMC article. Review.
Cited by
-
Dimethyl Fumarate ameliorates pulmonary arterial hypertension and lung fibrosis by targeting multiple pathways.Sci Rep. 2017 Feb 2;7:41605. doi: 10.1038/srep41605. Sci Rep. 2017. PMID: 28150703 Free PMC article.
-
Low let-7d exosomes from pulmonary vascular endothelial cells drive lung pericyte fibrosis through the TGFβRI/FoxM1/Smad/β-catenin pathway.J Cell Mol Med. 2020 Dec;24(23):13913-13926. doi: 10.1111/jcmm.15989. Epub 2020 Nov 12. J Cell Mol Med. 2020. PMID: 33179861 Free PMC article.
-
Alpinetin Suppresses Effects of TGF-β1 on Stimulating the Production and Organization of Fibrotic Markers in Human Primary Dermal Fibroblasts.Cells. 2022 Sep 1;11(17):2731. doi: 10.3390/cells11172731. Cells. 2022. PMID: 36078140 Free PMC article.
-
TGF-β signaling pathway mediated by deubiquitinating enzymes.Cell Mol Life Sci. 2019 Feb;76(4):653-665. doi: 10.1007/s00018-018-2949-y. Epub 2018 Oct 22. Cell Mol Life Sci. 2019. PMID: 30349992 Free PMC article. Review.
-
The Overexpression of TGF-β and CCN2 in Intrauterine Adhesions Involves the NF-κB Signaling Pathway.PLoS One. 2015 Dec 31;10(12):e0146159. doi: 10.1371/journal.pone.0146159. eCollection 2015. PLoS One. 2015. PMID: 26719893 Free PMC article.
References
-
- King TE. Update in pulmonary medicine. Ann Intern Med. 1998;129:806–812. - PubMed
-
- Raghu G, Freudenberger TD, Yang S, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136–142. - PubMed
-
- Marks JH. Update in pulmonary medicine. Adolesc Med State Art Rev. 2013;24:307–329. xvi. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

