MIF Receptor CD74 is Restricted to Microglia/Macrophages, Associated with a M1-Polarized Immune Milieu and Prolonged Patient Survival in Gliomas
- PMID: 25175718
- PMCID: PMC8029437
- DOI: 10.1111/bpa.12194
MIF Receptor CD74 is Restricted to Microglia/Macrophages, Associated with a M1-Polarized Immune Milieu and Prolonged Patient Survival in Gliomas
Abstract
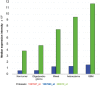
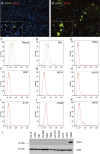
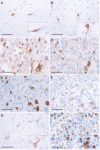
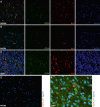
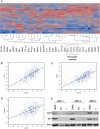
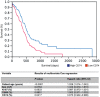
The macrophage migration inhibitory factor (MIF) receptor CD74 is overexpressed in various neoplasms, mainly in hematologic tumors, and currently investigated in clinical studies. CD74 is quickly internalized and recycles after antibody binding, therefore it constitutes an attractive target for antibody-based treatment strategies. CD74 has been further described as one of the most up-regulated molecules in human glioblastomas. To assess the potential relevance for anti-CD74 treatment, we determined the cellular source and clinicopathologic relevance of CD74 expression in human gliomas by immunohistochemistry, immunofluorescence, immunoblotting, cell sorting analysis and quantitative polymerase chain reaction (qPCR). Furthermore, we fractionated glioblastoma cells and glioma-associated microglia/macrophages (GAMs) from primary tumors and compared CD74 expression in cellular fractions with whole tumor lysates. Our results show that CD74 is restricted to GAMs in vivo, while being absent in tumor cells, the latter strongly expressing its ligand MIF. Most interestingly, a higher amount of CD74-positive GAMs was associated with beneficial patient survival constituting an independent prognostic parameter and with an anti-tumoral M1 polarization. In summary, CD74 expression in human gliomas is restricted to GAMs and positively associated with patient survival. In conclusion, CD74 represents a positive prognostic marker most probably because of its association with an M1-polarized immune milieu in high-grade gliomas.
Keywords: CD74; MIF; glioma; immune polarization.
© 2014 International Society of Neuropathology.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Al Abed Y, Dabideen D, Aljabari B, Valster A, Messmer D, Ochani M et al (2005) ISO‐1 binding to the tautomerase active site of MIF inhibits its pro‐inflammatory activity and increases survival in severe sepsis. J Biol Chem 280:36541–36544. - PubMed
-
- An JH, Lee SY, Jeon JY, Cho KG, Kim SU, Lee MA (2009) Identification of gliotropic factors that induce human stem cell migration to malignant tumor. J Proteome Res 8:2873–2881. - PubMed
-
- Apte RS, Sinha D, Mayhew E, Wistow GJ, Niederkorn JY (1998) Cutting edge: role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J Immunol 160:5693–5696. - PubMed
-
- Bacher M, Schrader J, Thompson N, Kuschela K, Gemsa D, Waeber G, Schlegel J (2003) Up‐regulation of macrophage migration inhibitory factor gene and protein expression in glial tumour cells during hypoxic and hypoglycemic stress indicates a critical role for angiogenesis in glioblastoma multiforme. Am J Pathol 162:11–17. - PMC - PubMed
-
- Baron N, Deuster O, Noelker C, Stüer C, Strik H, Schaller C et al (2011) Role of macrophage migration inhibitory factor in primary glioblastoma multiforme cells. J Neurosci Res 89:711–717. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

