Neuroimmune pathways in alcohol consumption: evidence from behavioral and genetic studies in rodents and humans
- PMID: 25175860
- PMCID: PMC4264574
- DOI: 10.1016/B978-0-12-801284-0.00002-6
Neuroimmune pathways in alcohol consumption: evidence from behavioral and genetic studies in rodents and humans
Abstract
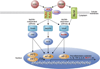
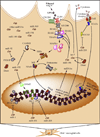
Immune or brain proinflammatory signaling has been linked to some of the behavioral effects of alcohol. Immune signaling appears to regulate voluntary ethanol intake in rodent models, and ethanol intake activates the immune system in multiple models. This bidirectional link raises the possibility that consumption increases immune signaling, which in turn further increases consumption in a feed-forward cycle. Data from animal and human studies provide overlapping support for the involvement of immune-related genes and proteins in alcohol action, and combining animal and human data is a promising approach to systematically evaluate and nominate relevant pathways. Based on rodent models, neuroimmune pathways may represent unexplored, nontraditional targets for medication development to reduce alcohol consumption and prevent relapse. Peroxisome proliferator-activated receptor agonists are one class of anti-inflammatory medications that demonstrate antiaddictive properties for alcohol and other drugs of abuse. Expression of immune-related genes is altered in animals and humans following chronic alcohol exposure, and the regulatory influences of specific mRNAs, microRNAs, and activated cell types are areas of intense study. Ultimately, the use of multiple datasets combined with behavioral validation will be needed to link specific neuroimmune pathways to addiction vulnerability.
Keywords: Dependence; Ethanol; Gene expression; Human alcoholics; Knock-out mice; LPS; PPAR; Preference; TLR4; Two-bottle choice; mRNA; microRNA.
© 2014 Elsevier Inc. All rights reserved.
Figures

References
-
- Agrawal RG, Owen JA, Levin PS, Hewetson A, Berman AE, Franklin SR, et al. Bioinformatics analyses reveal age-specific neuroimmune modulation as a target for treatment of high ethanol drinking. Alcoholism, Clinical and Experimental Research. 2014;38(2):428–437. http://dx.doi.org/10.1111/acer.12288. - DOI - PMC - PubMed
-
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. The Journal of Neuroscience. 2010;30(24):8285–8295. http://dx.doi.org/10.1523/JNEUROSCI.0976-10.2010. - DOI - PMC - PubMed
-
- Ambros V. microRNAs: Tiny regulators with great potential. Cell. 2001;107(7):823–826. - PubMed
-
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, et al. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha) The Journal of Biological Chemistry. 1998;273(10):5678–5684. - PubMed
-
- Baek MN, Jung KH, Halder D, Choi MR, Lee BH, Lee BC, et al. Artificial microRNA-based neurokinin-1 receptor gene silencing reduces alcohol consumption in mice. Neuroscience Letters. 2010;475(3):124–128. http://dx.doi.org/10.1016/j.neulet.2010.03.051. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

