Cloning and expression of porcine β1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen
- PMID: 25176027
- PMCID: PMC4262693
- DOI: 10.1111/xen.12124
Cloning and expression of porcine β1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen
Abstract
Background: Xenograft rejection of pigs organs with an engineered mutation in the GGTA-1 gene (GTKO) remains a predominantly antibody mediated process which is directed to a variety of non-Gal protein and carbohydrate antigens. We previously used an expression library screening strategy to identify six porcine endothelial cell cDNAs which encode pig antigens that bind to IgG induced after pig-to-primate cardiac xenotransplantation. One of these gene products was a glycosyltransferase with homology to the bovine β1,4 N-acetylgalactosaminyltransferase (B4GALNT2). We now characterize the porcine B4GALNT2 gene sequence, genomic organization, expression, and functional significance.
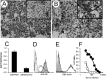
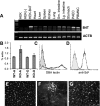
Methods: The porcine B4GALNT2 cDNA was recovered from the original library isolate, subcloned, sequenced, and used to identify a bacterial artificial chromosome (BAC) containing the entire B4GALNT2 locus from the Children's Hospital Oakland Research Institute BACPAC Resource Centre (#AC173453). PCR primers were designed to map the intron/exon genomic organization in the BAC clone. A stable human embryonic kidney (HEK) cell line expressing porcine B4GALNT2 (HEK-B4T) was produced. Expression of porcine B4GALNT2 in HEK-B4T cells was characterized by immune staining and siRNA transfection. The effects of B4GALNT2 expression in HEK-B4T cells was measured by flow cytometry and complement mediated lysis. Antibody binding to HEK and HEK-B4T cells was used to detect an induced antibody response to the B4GALNT2 produced glycan and the results were compared to GTKO PAEC specific non-Gal antibody induction. Expression of porcine B4GALNT2 in pig cells and tissues was measured by qualitative and quantitative real time reverse transcriptase PCR and by Dolichos biflorus agglutinin (DBA) tissue staining.
Results: The porcine B4GALNT2 gene shares a conserved genomic organization and encodes an open reading frame with 76 and 70% amino acid identity to the human and murine B4GALNT2 genes, respectively. The B4GALNT2 gene is expressed in porcine endothelial cells and shows a broadly distributed expression pattern. Expression of porcine B4GALNT2 in human HEK cells (HEK-B4T) results in increased binding of antibody to the B4GALNT2 enzyme, and increased reactivity with anti-Sd(a) and DBA. HEK-B4T cells show increased sensitivity to complement mediated lysis when challenged with serum from primates after pig to primate cardiac xenotransplantation. In GTKO and GTKO:CD55 cardiac xenotransplantation recipients there is a significant correlation between the induction of a non-Gal antibody, measured using GTKO PAECs, and the induction of antibodies which preferentially bind to HEK-B4T cells.
Conclusion: The functional isolation of the porcine B4GALNT2 gene from a PAEC expression library, the pattern of B4GALNT2 gene expression and its sensitization of HEK-B4T cells to antibody binding and complement mediated lysis indicates that the enzymatic activity of porcine B4GALNT2 produces a new immunogenic non-Gal glycan which contributes in part to the non-Gal immune response detected after pig-to-baboon cardiac xenotransplantation.
Keywords: B4GALNT2; antigen; cardiac xenotransplantation; immune response; porcine.
© 2014 The Authors. Xenotransplantation Published by John Wiley & Sons Ltd.
Figures



References
-
- Galili U, Shohet SB, Kobrin E, et al. Man, apes, and old world monkeys differ from other mammals in the expression of alpha-Galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762. - PubMed
-
- McGregor CG, Carpentier A, Lila N, et al. Cardiac xenotransplantation technology provides materials for improved bioprosthetic heart valves. J Thorac Cardiovasc Surg. 2011;141:269–275. - PubMed
-
- Manji RA, Menkis AH, Ekser B, Cooper DK. Porcine bioprosthetic heart valves: the next generation. Am Heart J. 2012;164:177–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

