Development of a consensus protocol to quantify primate anti-non-Gal xenoreactive antibodies using pig aortic endothelial cells
- PMID: 25176173
- PMCID: PMC4262663
- DOI: 10.1111/xen.12125
Development of a consensus protocol to quantify primate anti-non-Gal xenoreactive antibodies using pig aortic endothelial cells
Abstract
Background: Scientists working in the field of xenotransplantation do not employ a uniform method to measure and report natural and induced antibody responses to non-Galα(1,3)Gal (non-Gal) epitopes. Such humoral responses are thought to be particularly pathogenic after transplantation of vascularized GalTKO pig organs and having a more uniform assay and reporting format would greatly facilitate comparisons between laboratories.
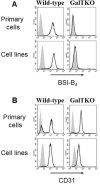
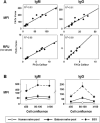
Methods: Flow cytometry allows examination of antibody reactivity to intact antigens in their natural location and conformation on cell membranes. We have established a simple and reproducible flow cytometric assay to detect antibodies specific for non-Gal pig antigens using primary porcine aortic endothelial cells (pAECs) and cell culture-adapted pAEC cell lines generated from wild type and α1,3galactosyl transferase knockout (GalTKO) swine.
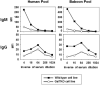
Results: The consensus protocol we propose here is based on procedures routinely used in four xenotransplantation centers and was independently evaluated at three sites using shared cells and serum samples. Our observation support use of the cell culture-adapted GalTKO pAEC KO:15502 cells as a routine method to determine the reactivity of anti-non-Gal antibodies in human and baboon serum.
Conclusions: We have developed an assay that allows the detection of natural and induced non-Gal xenoreactive antibodies present in human or baboon serum in a reliable and consistent manner. This consensus assay and format for reporting the data should be accessible to laboratories and will be useful for assessing experimental results between multiple research centers. Adopting this assay and format for reporting the data should facilitate the detection, monitoring, and detailed characterization of non-Gal antibody responses.
Keywords: antibody; cell line; galactosyl transferase; non-Gal antibody; xenoreactive assay; xenotransplantation.
© 2014 John Wiley & Sons A/S Published by John Wiley & Sons Ltd.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

