Panobinostat synergizes with bortezomib to induce endoplasmic reticulum stress and ubiquitinated protein accumulation in renal cancer cells
- PMID: 25176354
- PMCID: PMC4153447
- DOI: 10.1186/1471-2490-14-71
Panobinostat synergizes with bortezomib to induce endoplasmic reticulum stress and ubiquitinated protein accumulation in renal cancer cells
Abstract
Background: Inducing endoplasmic reticulum (ER) stress is a novel strategy used to treat malignancies. Inhibition of histone deacetylase (HDAC) 6 by the HDAC inhibitor panobinostat hinders the refolding of unfolded proteins by increasing the acetylation of heat shock protein 90. We investigated whether combining panobinostat with the proteasome inhibitor bortezomib would kill cancer cells effectively by inhibiting the degradation of these unfolded proteins, thereby causing ubiquitinated proteins to accumulate and induce ER stress.
Methods: Caki-1, ACHN, and 769-P cells were treated with panobinostat and/or bortezomib. Cell viability, clonogenicity, and induction of apoptosis were evaluated. The in vivo efficacy of the combination was evaluated using a murine subcutaneous xenograft model. The combination-induced ER stress and ubiquitinated protein accumulation were assessed.
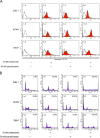
Results: The combination of panobinostat and bortezomib induced apoptosis and inhibited renal cancer growth synergistically (combination indexes <1). It also suppressed colony formation significantly (p <0.05). In a murine subcutaneous tumor model, a 10-day treatment was well tolerated and inhibited tumor growth significantly (p <0.05). Enhanced acetylation of the HDAC6 substrate alpha-tubulin was consistent with the suppression of HDAC6 activity by panobinostat, and the combination was shown to induce ER stress and ubiquitinated protein accumulation synergistically.
Conclusions: Panobinostat inhibits renal cancer growth by synergizing with bortezomib to induce ER stress and ubiquitinated protein accumulation. The current study provides a basis for testing the combination in patients with advanced renal cancer.
Figures




References
-
- Mimnaugh EG, Xu W, Vos M, Yuan X, Isaacs JS, Bisht KS, Gius D, Neckers L. Simultaneous inhibition of hsp 90 and the proteasome promotes protein ubiquitination, causes endoplasmic reticulum-derived cytosolic vacuolization, and enhances antitumor activity. Mol Cancer Ther. 2004;3:551–566. - PubMed
-
- Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, Seto E, Bhalla K. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005;280:26729–26734. doi: 10.1074/jbc.C500186200. - DOI - PubMed
-
- Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

