Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging
- PMID: 25176656
- PMCID: PMC4534571
- DOI: 10.1016/j.celrep.2014.07.061
Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging
Abstract
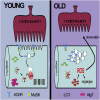
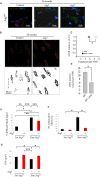
The cellular basis of age-related tissue deterioration remains largely obscure. The ability to activate compensatory mechanisms in response to environmental stress is an important factor for survival and maintenance of cellular functions. Autophagy is activated both under short and prolonged stress and is required to clear the cell of dysfunctional organelles and altered proteins. We report that specific autophagy inhibition in muscle has a major impact on neuromuscular synaptic function and, consequently, on muscle strength, ultimately affecting the lifespan of animals. Inhibition of autophagy also exacerbates aging phenotypes in muscle, such as mitochondrial dysfunction, oxidative stress, and profound weakness. Mitochondrial dysfunction and oxidative stress directly affect acto-myosin interaction and force generation but show a limited effect on stability of neuromuscular synapses. These results demonstrate that age-related deterioration of synaptic structure and function is exacerbated by defective autophagy.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Blaauw B., Mammucari C., Toniolo L., Agatea L., Abraham R., Sandri M., Reggiani C., Schiaffino S. Akt activation prevents the force drop induced by eccentric contractions in dystrophin-deficient skeletal muscle. Hum. Mol. Genet. 2008;17:3686–3696. - PubMed
-
- Bottinelli R., Pellegrino M.A., Canepari M., Rossi R., Reggiani C. Specific contributions of various muscle fibre types to human muscle performance: an in vitro study. J. Electromyogr. Kinesiol. 1999;9:87–95. - PubMed
-
- Canepari M., Rossi R., Pellegrino M.A., Bottinelli R., Schiaffino S., Reggiani C. Functional diversity between orthologous myosins with minimal sequence diversity. J. Muscle Res. Cell Motil. 2000;21:375–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

