Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†
- PMID: 25176939
- PMCID: PMC4359359
- DOI: 10.1093/eurheartj/ehu385
Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†
Abstract
Aim: The aim of this study was to evaluate the benefits and safety of long-term i.v. iron therapy in iron-deficient patients with heart failure (HF).
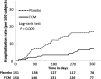
Methods and results: CONFIRM-HF was a multi-centre, double-blind, placebo-controlled trial that enrolled 304 ambulatory symptomatic HF patients with left ventricular ejection fraction ≤45%, elevated natriuretic peptides, and iron deficiency (ferritin <100 ng/mL or 100-300 ng/mL if transferrin saturation <20%). Patients were randomized 1 : 1 to treatment with i.v. iron, as ferric carboxymaltose (FCM, n = 152) or placebo (saline, n = 152) for 52 weeks. The primary end-point was the change in 6-min-walk-test (6MWT) distance from baseline to Week 24. Secondary end-points included changes in New York Heart Association (NYHA) class, Patient Global Assessment (PGA), 6MWT distance, health-related quality of life (QoL), Fatigue Score at Weeks 6, 12, 24, 36, and 52 and the effect of FCM on the rate of hospitalization for worsening HF. Treatment with FCM significantly prolonged 6MWT distance at Week 24 (difference FCM vs. placebo: 33 ± 11 m, P = 0.002). The treatment effect of FCM was consistent in all subgroups and was sustained to Week 52 (difference FCM vs. placebo: 36 ± 11 m, P < 0.001). Throughout the study, an improvement in NYHA class, PGA, QoL, and Fatigue Score in patients treated with FCM was detected with statistical significance observed from Week 24 onwards. Treatment with FCM was associated with a significant reduction in the risk of hospitalizations for worsening HF [hazard ratio (95% confidence interval): 0.39 (0.19-0.82), P = 0.009]. The number of deaths (FCM: 12, placebo: 14 deaths) and the incidence of adverse events were comparable between both groups.
Conclusion: Treatment of symptomatic, iron-deficient HF patients with FCM over a 1-year period resulted in sustainable improvement in functional capacity, symptoms, and QoL and may be associated with risk reduction of hospitalization for worsening HF (ClinicalTrials.gov number NCT01453608).
Keywords: Ferric carboxymaltose; Heart failure; Iron deficiency.
© The Author 2014. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



Comment in
-
Heart failure: Long-term iron therapy is beneficial in patients with HF.Nat Rev Cardiol. 2014 Nov;11(11):622. doi: 10.1038/nrcardio.2014.147. Epub 2014 Sep 16. Nat Rev Cardiol. 2014. PMID: 25223446 No abstract available.
-
Iron i.v. in heart failure: ready for implementation?Eur Heart J. 2015 Mar 14;36(11):645-7. doi: 10.1093/eurheartj/ehu392. Epub 2014 Oct 21. Eur Heart J. 2015. PMID: 25336211 Free PMC article. No abstract available.
References
-
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. - PubMed
-
- Tavazzi L, Senni M, Metra M, Gorini M, Cacciatore G, Chinaglia A, Di Lenarda A, Mortara A, Oliva F, Maggioni AP IN-HF (Italian Network on Heart Failure) Outcome Investigators. Multicenter prospective observational study on acute and chronic heart failure: one-year follow-up results of IN-HF (Italian Network on Heart Failure) outcome registry. Circ Heart Fail. 2013;6:473–481. - PubMed
-
- Hoekstra T, Jaarsma T, van Veldhuisen DJ, Jillege HL, Sanderman R, Lesman-Leegte I. Quality of life and survival in patients with heart failure. Eur J Heart Fail. 2013;15:94–102. - PubMed
-
- Van Deursen VM, Urso R, Laroche C, Damman K, Dahlstrom U, Tavazzi L, Maggioni AP, Voors AA. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail. 2014;16:103–111. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

