Peroxisome biogenesis in mammalian cells
- PMID: 25177298
- PMCID: PMC4133648
- DOI: 10.3389/fphys.2014.00307
Peroxisome biogenesis in mammalian cells
Abstract
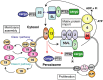
To investigate peroxisome assembly and human peroxisome biogenesis disorders (PBDs) such as Zellweger syndrome, thirteen different complementation groups (CGs) of Chinese hamster ovary (CHO) cell mutants defective in peroxisome biogenesis have been isolated and established as a model research system. Successful gene-cloning studies by a forward genetic approach utilized a rapid functional complementation assay of CHO cell mutants led to isolation of human peroxin (PEX) genes. Search for pathogenic genes responsible for PBDs of all 14 CGs is now completed together with the homology search by screening the human expressed sequence tag database using yeast PEX genes. Peroxins are divided into three groups: (1) peroxins including Pex3p, Pex16p, and Pex19p, are responsible for peroxisome membrane biogenesis via classes I and II pathways; (2) peroxins that function in matrix protein import; (3) those such as three forms of Pex11p, Pex11pα, Pex11pβ, and Pex11pγ, are involved in peroxisome proliferation where DLP1, Mff, and Fis1 coordinately function. In membrane assembly, Pex19p forms complexes in the cytosol with newly synthesized PMPs including Pex16p and transports them to the receptor Pex3p, whereby peroxisomal membrane is formed (Class I pathway). Pex19p likewise forms a complex with newly made Pex3p and translocates it to the Pex3p receptor, Pex16p (Class II pathway). In matrix protein import, newly synthesized proteins harboring peroxisome targeting signal type 1 or 2 are recognized by Pex5p or Pex7p in the cytoplasm and are imported to peroxisomes via translocation machinery. In regard to peroxisome-cytoplasmic shuttling of Pex5p, Pex5p initially targets to an 800-kDa docking complex consisting of Pex14p and Pex13p and then translocates to a 500-kDa RING translocation complex. At the terminal step, Pex1p and Pex6p of the AAA family mediate the export of Pex5p, where Cys-ubiquitination of Pex5p is essential for the Pex5p exit.
Keywords: CHO cell mutants; Zellweger syndrome; genetic phenotype-complementation; import machinery; membrane assembly; pathogenic genes; peroxins; peroxisome targeting signals.
Figures


References
-
- Baerends R. J. S., Rasmussen S. W., Hilbrands R. E., Van Der Heide M., Faber K. N., Reuvekamp P. T. W., et al. (1996). The Hansenula polymorpha PER9 gene encodes a peroxisomal membrane protein essential for peroxisome assembly and integrity. J. Biol. Chem. 271, 8887–8894 10.1074/jbc.271.15.8887 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

