Bioinformatic Analysis of Differential Protein Expression in Calu-3 Cells Exposed to Carbon Nanotubes
- PMID: 25177543
- PMCID: PMC4148817
- DOI: 10.3390/proteomes1030219
Bioinformatic Analysis of Differential Protein Expression in Calu-3 Cells Exposed to Carbon Nanotubes
Abstract
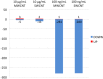
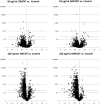
Carbon nanomaterials are widely produced and used in industry, medicine and scientific research. To examine the impact of exposure to nanoparticles on human health, the human airway epithelial cell line, Calu-3, was used to evaluate changes in the cellular proteome that could account for alterations in cellular function of airway epithelia after 24 hexposure to 10 μg/mL and 100 ng/mL of two common carbon nanoparticles, single- and multi-wall carbon nanotubes (SWCNT, MWCNT). After exposure to the nanoparticles, label-free quantitative mass spectrometry (LFQMS) was used to study the differential protein expression. Ingenuity Pathway Analysis (IPA) was used to conduct a bioinformaticanalysis of proteins identified in LFQMS. Interestingly, after exposure to ahigh concentration (10 μg/mL; 0.4 μg/cm2) of MWCNT or SWCNT, only 8 and 13 proteins, respectively, exhibited changes in abundance. In contrast, the abundance of hundreds of proteins was altered in response to a low concentration (100 ng/mL; 4 ng/cm2) of either CNT. Of the 281 and 282 proteins that were significantly altered in response to MWCNT or SWCNT respectively, 231 proteins were the same. Bioinformatic analyses found that the proteins in common to both nanotubes occurred within the cellular functions of cell death and survival, cell-to-cell signaling and interaction, cellular assembly and organization, cellular growth and proliferation, infectious disease, molecular transport and protein synthesis. The majority of the protein changes represent a decrease in amount suggesting a general stress response to protect cells. The STRING database was used to analyze the various functional protein networks. Interestingly, some proteins like cadherin 1 (CDH1), signal transducer and activator of transcription 1 (STAT1), junction plakoglobin (JUP), and apoptosis-associated speck-like protein containing a CARD (PYCARD), appear in several functional categories and tend to be in the center of the networks. This central positioning suggests they may play important roles in multiple cellular functions and activities that are altered in response to carbon nanotube exposure.
Keywords: airway epithelia; barrier epithelia; label-free quantitative mass spectrometry; protein interaction networks.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Functional effects of nanoparticle exposure on Calu-3 airway epithelial cells.Cell Physiol Biochem. 2012;29(1-2):197-212. doi: 10.1159/000337601. Epub 2012 Mar 1. Cell Physiol Biochem. 2012. PMID: 22415089 Free PMC article.
-
Protein expression profiles of intestinal epithelial co-cultures: effect of functionalised carbon nanotube exposure.Int J Biomed Nanosci Nanotechnol. 2013;3(1-2):10.1504/IJBNN.2013.054508. doi: 10.1504/IJBNN.2013.054508. Int J Biomed Nanosci Nanotechnol. 2013. PMID: 24228069 Free PMC article.
-
Effects of fullerene (C60), multi-wall carbon nanotubes (MWCNT), single wall carbon nanotubes (SWCNT) and hydroxyl and carboxyl modified single wall carbon nanotubes on riverine microbial communities.Environ Sci Pollut Res Int. 2016 May;23(10):10090-102. doi: 10.1007/s11356-016-6244-x. Epub 2016 Feb 12. Environ Sci Pollut Res Int. 2016. PMID: 26867687
-
Imogolite: an aluminosilicate nanotube endowed with low cytotoxicity and genotoxicity.Chem Res Toxicol. 2014 Jul 21;27(7):1142-54. doi: 10.1021/tx500002d. Epub 2014 Jun 16. Chem Res Toxicol. 2014. PMID: 24933079
-
Single-walled and multi-walled carbon nanotubes induce sequence-specific epigenetic alterations in 16 HBE cells.Oncotarget. 2018 Apr 17;9(29):20351-20365. doi: 10.18632/oncotarget.24866. eCollection 2018 Apr 17. Oncotarget. 2018. PMID: 29755656 Free PMC article.
References
-
- Correa-Duarte M.A., Wagner N., Rojas-Chapana J., Morsczeck C., Thie M., Giersig M. Fabrication and biocompatibility of carbon nanotube-based 3D networks as scaffolds for cell seeding and growth. Nano Lett. 2004;4:2233–2236. doi: 10.1021/nl048574f. - DOI
-
- Wong S.K.N., Dai H. Single walled carbon nanotubes for transport and delivery of biological cargos. Physica Status Solidi B. 2006;243:3561–3566. doi: 10.1002/pssb.200669226. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
