A novel, ultrasensitive assay for tau: potential for assessing traumatic brain injury in tissues and biofluids
- PMID: 25177776
- PMCID: PMC4348038
- DOI: 10.1089/neu.2014.3548
A novel, ultrasensitive assay for tau: potential for assessing traumatic brain injury in tissues and biofluids
Abstract
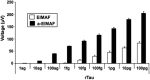
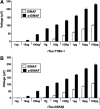
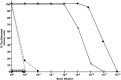
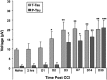
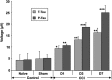
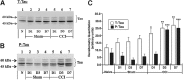
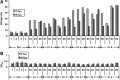
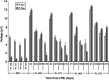
Traumatic brain injury (TBI) is a cause of death and disability and can lead to tauopathy-related dementia at an early age. Pathologically, TBI results in axonal injury that is coupled to tau hyperphosphorylation, leading to microtubule instability and tau-mediated neurodegeneration. This suggests that the forms of this protein might serve as neuroinjury-related biomarkers for diagnosis of injury severity and prognosis of the neurological damage prior to clinical expression. We initially determined whether we could detect tau in body fluids using a highly sensitive assay. We used a novel immunoassay, enhanced immunoassay using multi-arrayed fiberoptics (EIMAF) either alone or in combination with rolling circle amplification (a-EIMAF) for the detection of total (T) and phosphorylated (P) tau proteins from brains and biofluids (blood, CSF) of rodents following controlled cortical impact (CCI) and human patients post severe TBI (sTBI). This assay technology for tau is the most sensitive to date with a detection limit of approximately 100 ag/mL for either T-tau and P-tau. In the rodent models, T-tau and P-tau levels in brain and blood increased following CCI during the acute phase and remained high during the chronic phase (30 d). In human CSF samples, T-tau and P-tau increased during the sampling period (5-6 d). T-tau and P-tau in human serum rose during the acute phase and decreased during the chronic stage but was still detectable beyond six months post sTBI. Thus, EIMAF has the potential for assessing both the severity of the proximal injury and the prognosis using easily accessible samples.
Keywords: EIMAF; brain and biofluids; rolling circle amplification; severe traumatic brain injury; total and phosphorylated tau.
Figures
Similar articles
-
Temporal Profiles of P-Tau, T-Tau, and P-Tau:Tau Ratios in Cerebrospinal Fluid and Blood from Moderate-Severe Traumatic Brain Injury Patients and Relationship to 6-12 Month Global Outcomes.J Neurotrauma. 2024 Feb;41(3-4):369-392. doi: 10.1089/neu.2022.0479. Epub 2023 Nov 16. J Neurotrauma. 2024. PMID: 37725589
-
Comparing Plasma Phospho Tau, Total Tau, and Phospho Tau-Total Tau Ratio as Acute and Chronic Traumatic Brain Injury Biomarkers.JAMA Neurol. 2017 Sep 1;74(9):1063-1072. doi: 10.1001/jamaneurol.2017.0655. JAMA Neurol. 2017. PMID: 28738126 Free PMC article.
-
Neurobiochemical, Peptidomic, and Bioinformatic Approaches to Characterize Tauopathy Peptidome Biomarker Candidates in Experimental Mouse Model of Traumatic Brain Injury.Mol Neurobiol. 2023 Apr;60(4):2295-2319. doi: 10.1007/s12035-022-03165-y. Epub 2023 Jan 13. Mol Neurobiol. 2023. PMID: 36635478
-
Traumatic brain injury (TBI) in collision sports: Possible mechanisms of transformation into chronic traumatic encephalopathy (CTE).Metabolism. 2019 Nov;100S:153943. doi: 10.1016/j.metabol.2019.07.007. Metabolism. 2019. PMID: 31610856 Review.
-
Does neuroinflammation drive the relationship between tau hyperphosphorylation and dementia development following traumatic brain injury?Brain Behav Immun. 2017 Feb;60:369-382. doi: 10.1016/j.bbi.2016.09.027. Epub 2016 Sep 28. Brain Behav Immun. 2017. PMID: 27686843 Review.
Cited by
-
Serum Cleaved Tau Protein and Clinical Outcome in Patients with Minor Head Trauma.Open Access Emerg Med. 2020 Jan 20;12:7-12. doi: 10.2147/OAEM.S217424. eCollection 2020. Open Access Emerg Med. 2020. PMID: 32021498 Free PMC article.
-
Penetrating Traumatic Brain Injury Triggers Dysregulation of Cathepsin B Protein Levels Independent of Cysteine Protease Activity in Brain and Cerebral Spinal Fluid.J Neurotrauma. 2020 Jul 1;37(13):1574-1586. doi: 10.1089/neu.2019.6537. Epub 2020 Apr 2. J Neurotrauma. 2020. PMID: 31973644 Free PMC article.
-
Exploiting blood-based biomarkers to align preclinical models with human traumatic brain injury.Brain. 2025 Apr 3;148(4):1062-1080. doi: 10.1093/brain/awae350. Brain. 2025. PMID: 39514789 Free PMC article.
-
CSF p-tau as a potential cognition impairment biomarker in ALS.Front Neurol. 2022 Nov 1;13:991143. doi: 10.3389/fneur.2022.991143. eCollection 2022. Front Neurol. 2022. Retraction in: Front Neurol. 2024 Mar 04;15:1392563. doi: 10.3389/fneur.2024.1392563. PMID: 36388201 Free PMC article. Retracted.
-
High-specificity antibodies and detection methods for quantifying phosphorylated tau from clinical samples.Antib Ther. 2021 Feb 15;4(1):34-44. doi: 10.1093/abt/tbab004. eCollection 2021 Jan. Antib Ther. 2021. PMID: 33928234 Free PMC article. Review.
References
-
- Chiu W.T., Huang S.J., Tsai S.H., Lin J-W., Tsai M-D., and Lin T-J. (2007). The impact of time, legislation, and geography on the epidemiology of traumatic brain injury. J. Clin. Neurosci. 14, 930–935 - PubMed
-
- Clinton J., Ambler M.W., and Roberts G.W. (1991). Post-traumatic Alzheimer's disease: preponderance of a single plaque type. Neuropathol. Appl. Neurobiol. 17, 69–74 - PubMed
-
- Mortimer J.A., Van Duijn C.M., Chandra V., Fratiglioni L., Graves A.B., Heyman A., Jorm A.F., Kokmen E., Rocca W.A., Shalat S.L., Soininen H., and Hofman A. (1991). Head trauma as a risk factor for Alzheimer's disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int. J. Epidemiol. 20, S28–S35 - PubMed
-
- Breteler M.M., Claus J.J., Van Duijn C.M., Launer L.J., and Hofman A. (1992). Epidemiology of Alzheimer's disease. Epidemiol Rev 14, 59–82 - PubMed
-
- Mayeux R., Ottman R., Tang M.X., Noboa-Bauza L., Marder K., Gurland B., and Stem Y. (1993). Genetic susceptibility and head injury as risk factors for Alzheimer's disease among community-dwelling elderly persons and their first-degree relatives. Ann. Neurol. 33, 494–501 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

