Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease
- PMID: 25178099
- PMCID: PMC11195634
- DOI: 10.1002/14651858.CD001288.pub4
Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease
Abstract
Background: Acute exacerbations of chronic obstructive pulmonary disease (COPD) are a major cause of hospital admission and mortality. They contribute to long-term decline in lung function, physical capacity and quality of life. The most common causes are infective, and treatment includes antibiotics, bronchodilators and systemic corticosteroids as anti-inflammatory agents.
Objectives: To assess the effects of corticosteroids administered orally or parenterally for treatment of acute exacerbations of COPD, and to compare the efficacy of parenteral versus oral administration.
Search methods: We carried out searches using the Cochrane Airways Group Specialised Register of Trials, MEDLINE and CENTRAL (Cochrane Central Register of Controlled Trials), and checked references of included studies and trials registries. We conducted the last search in May 2014.
Selection criteria: Randomised controlled trials comparing corticosteroids administered orally or parenterally with an appropriate placebo, or comparing oral corticosteroids with parenteral corticosteroids in the treatment of people with acute exacerbations of COPD. Other interventions (e.g. bronchodilators and antibiotics) were standardised for both groups. We excluded clinical studies of acute asthma.
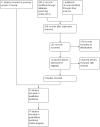
Data collection and analysis: We used standard methodological procedures expected by The Cochrane Collaboration.
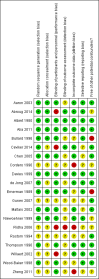
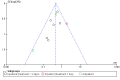
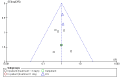
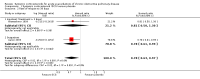
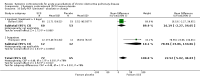
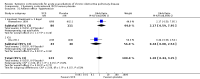
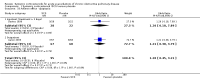
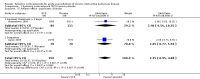
Main results: Sixteen studies (n = 1787) met inclusion criteria for the comparison systemic corticosteroid versus placebo and 13 studies contributed data (n = 1620). Four studies (n = 298) met inclusion criteria for the comparison oral corticosteroid versus parenteral corticosteroid and three studies contributed data (n = 239). The mean age of participants with COPD was 68 years, median proportion of males 82% and mean forced expiratory volume in one second (FEV1) per cent predicted at study admission was 40% (6 studies; n = 633). We judged risk of selection, detection, attrition and reporting bias as low or unclear in all studies. We judged risk of performance bias high in one study comparing systemic corticosteroid with control and in two studies comparing intravenous corticosteroid versus oral corticosteroid.Systemic corticosteroids reduced the risk of treatment failure by over half compared with placebo in nine studies (n = 917) with median treatment duration 14 days, odds ratio (OR) 0.48 (95% confidence interval (CI) 0.35 to 0.67). The evidence was graded as high quality and it would have been necessary to treat nine people (95% CI 7 to 14) with systemic corticosteroids to avoid one treatment failure. There was moderate-quality evidence for a lower rate of relapse by one month for treatment with systemic corticosteroid in two studies (n = 415) (hazard ratio (HR) 0.78; 95% CI 0.63 to 0.97). Mortality up to 30 days was not reduced by treatment with systemic corticosteroid compared with control in 12 studies (n = 1319; OR 1.00; 95% CI 0.60 to 1.66).FEV1, measured up to 72 hours, showed significant treatment benefits (7 studies; n = 649; mean difference (MD) 140 mL; 95% CI 90 to 200); however, this benefit was not observed at later time points. The likelihood of adverse events increased with corticosteroid treatment (OR 2.33; 95% CI 1.59 to 3.43). Overall, one extra adverse effect occurred for every six people treated (95% CI 4 to 10). The risk of hyperglycaemia was significantly increased (OR 2.79; 95% CI 1.86 to 4.19). For general inpatient treatment, duration of hospitalisation was significantly shorter with corticosteroid treatment (MD -1.22 days; 95% CI -2.26 to -0.18), with no difference in length of stay the intensive care unit (ICU) setting.Comparison of parenteral versus oral treatment showed no significant difference in the primary outcomes of treatment failure, relapse or mortality or for any secondary outcomes. There was a significantly increased rate of hyperglycaemia in one study (OR 4.89; 95% CI 1.20 to 19.94).
Authors' conclusions: There is high-quality evidence to support treatment of exacerbations of COPD with systemic corticosteroid by the oral or parenteral route in reducing the likelihood of treatment failure and relapse by one month, shortening length of stay in hospital inpatients not requiring assisted ventilation in ICU and giving earlier improvement in lung function and symptoms. There is no evidence of benefit for parenteral treatment compared with oral treatment with corticosteroid on treatment failure, relapse or mortality. There is an increase in adverse drug effects with corticosteroid treatment, which is greater with parenteral administration compared with oral treatment.
Conflict of interest statement
R Wood‐Baker has undertaken research sponsored by a number of pharmaceutical companies including Marion Merrell Dow, ICI Australia, Pharmacia Upjohn, GlaxoSmithKline, Sanofi‐Synthélabo and Novartis.
E H Walters has undertaken research sponsored by a number of pharmaceutical companies including Glaxo Wellcome, Novartis, Astra, Boehringer, Schering‐Plough and Amgen. He has held consultancies for AstraZeneca, ICI Australia, Pfizer and GlaxoSmithKline.
Figures
















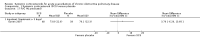



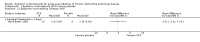



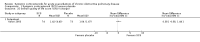
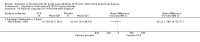















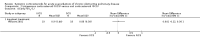


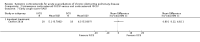
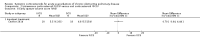
















Update of
-
Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2009 Jan 21;(1):CD001288. doi: 10.1002/14651858.CD001288.pub3. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2014 Sep 01;(9):CD001288. doi: 10.1002/14651858.CD001288.pub4. PMID: 19160195 Updated.
Comment in
-
[Analysis of the Cochrane Review: Use of systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014, 9:CD001288].Acta Med Port. 2014 Sep-Oct;27(5):537-40. Epub 2014 Oct 31. Acta Med Port. 2014. PMID: 25409206 Portuguese.
-
ACP journal club. Review: systemic corticosteroids reduce treatment failure but increase hyperglycemia in COPD exacerbations.Ann Intern Med. 2015 Mar 17;162(6):JC6- JC7. doi: 10.7326/ACPJC-2015-162-6-006. Ann Intern Med. 2015. PMID: 25775349 No abstract available.
-
Do Systemic Corticosteroids Improve Outcomes in Chronic Obstructive Pulmonary Disease Exacerbations?Ann Emerg Med. 2016 Feb;67(2):258-9. doi: 10.1016/j.annemergmed.2015.07.009. Epub 2015 Jul 23. Ann Emerg Med. 2016. PMID: 26210382 No abstract available.
References
References to studies included in this review
Aaron 2003 {published and unpublished data}
-
- Aaron SD, Vandemheen KL, Hebert P, Dales R, Stiell IG, Ahuja J, et al. Outpatient oral prednisone after emergency treatment of chronic obstructive pulmonary disease. New England Journal of Medicine 2003;348(26):2618‐25. - PubMed
-
- Aaron SD, Vandemheen KL, Wells G, Dales R, Ahuja J, Dickinson G, et al. Oral prednisone for the prevention of relapse in outpatients with acute exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 2003;167(7):A947.
-
- Courtney AU. Oral prednisone prevents relapse in COPD exacerbations. Journal of Family Practice 2003;52(10):762‐4. - PubMed
Abroug 2014 {published data only}
-
- Abroug F, Ouanes‐Besbes L, Fkih‐Hassen M, Ouanes I, Ayed S, Dachraoui F, et al. Prednisone in COPD exacerbation requiring ventilatory support: an open‐label randomised evaluation. European Respiratory Journal 2014;43(3):717‐24. - PubMed
Albert 1980 {published data only}
-
- Albert R, Martin T, Lewis S. Controlled clinical trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Annals of Internal Medicine 1980;92:753‐8. - PubMed
Alia 2011 {published and unpublished data}
-
- Alia I, Cal MA, Esteban A, Abella A, Ferrer R, Molina FJ, et al. Efficacy of corticosteroid therapy in patients with an acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support. Archives of Internal Medicine 2011;171(21):1939‐46. - PubMed
-
- Alia I, Cal MA, Esteban A, Ferrer R, Molina F, Pier R, et al. Evaluation of the efficacy of corticosteroids in patients with an acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support: a prospective, international, randomized double blind clinical trial. Intensive Care Medicine 2010;36(Suppl 2):S205.
Bullard 1996 {published and unpublished data}
-
- Bullard M, Liaw S‐J, Tsai Y‐H, Min H. Early corticosteroid use in acute exacerbations of chronic airflow obstruction. American Journal of Emergency Medicine 1996;14:139‐43. - PubMed
Ceviker 2014 {published data only (unpublished sought but not used)}
-
- Ceviker Y, Sayiner A. Comparison of two systemic steroid regimens for the treatment of COPD exacerbations. Pulmonary Pharmacology and Therapeutics 2014;27(2):179‐83. - PubMed
Chen 2005 {published and unpublished data}
-
- Chen G, Xie C. The efficacy and treatment length of oral corticosteroids in patients with acute exacerbations of chronic obstructive pulmonary disease. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1966.
-
- Chen G, Xie CM, Luo YF. The effects and therapeutic duration of oral corticosteroids in patients with acute exacerbation of chronic obstructive pulmonary diseases [In Chinese]. Chinese Journal of Tuberculosis and Respiratory Diseases 2008;31(8):577‐80. - PubMed
Cordero 1996 {published data only (unpublished sought but not used)}
-
- Cordero PJ, Benlloch E, SolÈ A, Morales P, MenÈndez R, Cebrian J. Corticosteroid therapy for COPD exacerbations at inpatient settings: a controlled‐randomized study. Archivos de Bronconeumologia 1996;32(Suppl 2):17.
-
- Cordero PJ, Sole A, Benlloch E, Morales P, Vallterra J, Menendez R, et al. A randomized controlled study of prednisone in outpatients with acute exacerbation of COPD. European Respiratory Journal 1996;9 Suppl 23:110s‐1s.
Davies 1999 {published and unpublished data}
-
- Davies L, Angus RM, Calverley PMA. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999;354:456‐60. - PubMed
de Jong 2007 {published data only}
-
- Jong Y, Uil M, Grotjohan P, Postma S, Kerstjens M, Berg J. A comparison of intravenous versus oral administration of corticosteroids in the treatment of patients admitted to the hospital with an exacerbation of COPD. European Respiratory Journal 2004;24(Suppl 48):64s.
-
- Jong YP, Uil SM, Grotjohan HP, Postma DS, Kerstjens HA, Berg JW. Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double‐blind study. Chest 2007;132(6):1741‐7. - PubMed
Emerman 1989 {published and unpublished data}
-
- Emerman C, Connors A, Lukens T, May M, Effron D. A randomized controlled trial of methylprednisolone in the emergency treatment of acute exacerbations of COPD. Chest 1989;95:563‐7. - PubMed
Gunen 2007 {published data only (unpublished sought but not used)}
-
- Gunen H, Hacievliyagil SS, Yetkin O, Gulbas G. The role of nebulised budesonide in the treatment of acute exacerbations of COPD. European Respiratory Journal 2007;30(2):399‐400. - PubMed
-
- Gunen H, Hacievliyagil SS, Yetkin O, Gulbas G, Mutlu LC, In E. The role of nebulised budesonide in the treatment of exacerbations of COPD. European Respiratory Journal 2007;29(4):660‐7. - PubMed
Maltais 2002 {published and unpublished data}
-
- Maltais F, Ostinelli J, Bourbeau J, Tonnel AB, Jacquemet N, Haddon J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2002;165(5):698‐703. - PubMed
Niewoehner 1999 {published and unpublished data}
-
- Erbland ML, Deupree RH, Niewoehner DE. Systemic corticosteroids in chronic obstructive pulmonary disease exacerbations (SCCOPE): rationale and design of an equivalence trial. Veterans Administration Cooperative Trials SCCOPE Study Group. Controlled Clinical Trials 1998;19(4):404‐17. - PubMed
-
- Gross N. Is there a subgroup of steroid responders in acute exacerbations of COPD (AECOPD)? Further evaluation of SCCOPE trial data. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A271.
-
- Niewoehner DE, Collins D, Erbland ML. Relation of FEV(1) to clinical outcomes during exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;161(4 pt1):1201‐5. - PubMed
-
- Niewoehner DE, Erbland ML, Duepree RH, Collins D, Gross NJ, Light RW, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. New England Journal of Medicine 1999;340:1941‐7. - PubMed
Ridha 2006 {published data only (unpublished sought but not used)}
-
- Ridha M, Ferdaous Y, Sofia T, Amel C, Ali BK. Effect of systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. European Respiratory Journal 2006;28(Suppl 50):624s.
Rostom 1994 {published data only}
-
- Rostom R, Mink S, Hebert P, Cardinal P. The long term efficacy of methylprednisolone in the treatment of acute exacerbation of COPD. Chest 1994;106(2):161S.
Thompson 1996 {published and unpublished data}
-
- Thompson W, Nielson C, Carvalho P, Charan N, Crowley J. Controlled trial of oral prednisolone in outpatients with acute COPD exacerbation. American Journal of Respiratory and Critical Care Medicine 1996;154:407‐12. - PubMed
Willaert 2002 {published data only}
-
- Willaert W, Daenen M, Bomans P, Verleden G, Decramer M. Intravenous steroids and aerosol bronchodilators versus oral steroids and MDI bronchodilators in COPD exacerbations. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A578.
-
- Willaert W, Daenen M, Bomans P, Verleden G, Decramer M. What is the optimal treatment strategy for chronic obstructive pulmonary disease exacerbations?. European Respiratory Journal 2002;19:928‐35. - PubMed
Wood‐Baker 1998 {published and unpublished data}
-
- Wood‐Baker R, Wilkinson J, Pearce M, Ryan G. A double‐blind, randomised, placebo‐controlled trial of corticosteroids for acute exacerbations of chronic obstructive pulmonary disease [Abstract]. Australian and New Zealand Journal of Medicine 1998;28:262.
Zheng 2011 {published data only (unpublished sought but not used)}
-
- Zheng J, Lin J, Zhou X, Huang S, Xie C, Liang Z, et al. Nebulized budesonide in the treatment of acute exacerbations of chronic obstructive pulmonary disease (AECOPD): a randomized, double blind, double dummy, parallel controlled, multicenter trial. Chest 2011;140(4):526A.
References to studies excluded from this review
Bafadhel 2012 {published data only}
-
- Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Lomas D, et al. A double‐blind randomised control trial of peripheral blood eosinophils to direct prednisolone use in COPD exacerbations. European Respiratory Journal 2011;38(55):38s [357].
-
- Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo‐controlled trial. American Journal of Respiratory and Critical Care Medicine 2012;186(1):48‐55. - PMC - PubMed
Bingol 2005 {published data only}
-
- Bingol H, Cingoz F, Balkan A, Kilic S, Bolcal C, Demirkilic U. The effect of oral prednisolone with chronic obstructive pulmonary disease undergoing coronary artery bypass surgery. Journal of Cardiac Surgery 2005;20:252‐6. - PubMed
Dahlen 2001 {published data only}
-
- Dahlen I, Janson C, Björsson E, Stålenheim G, Peterson CG, Venge P. Changes in inflammatory markers following treatment of acute exacerbations of obstructive pulmonary disease. Respiratory Medicine 2001;95:891‐7. - PubMed
Gerogianni 2002 {published data only}
-
- Gerogianni A, Mathioudakis G, Koulouris N, Orphanidou D, Malamos N, Markozannes E, et al. The effect of oral steroid treatment in patients with chronic bronchitis. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A594.
Ghanei 2005 {published data only}
-
- Ghanei M, Khalili A, Arab M, Mojtahedzadeh M, Aslani J, Lessan‐Pezeshki M, et al. Diagnostic and therapeutic value of short‐term corticosteroid therapy in exacerbation of mustard gas‐induced chronic bronchitis. Basic & Clinical Pharmacology and Toxicology 2005;97(5):302‐5. - PubMed
GSK FLIT98 {unpublished data only}
-
- GSK FLIT98. Inhaled fluticasone propionate in an acute exacerbation of chronic obstructive pulmonary disease: a multi‐centre, randomised, double‐blind, parallel group study of the efficacy and safety of inhaled fluticasone propionate 2000 µg daily compared with placebo in chronic obstructive pulmonary disease (chronic bronchitis). GlaxoSmithKline Clinical Trial Register 2005.
Kunter 2006 {published data only}
-
- Ilvan A, Kunter E, Demirer E, Avsar K, Kartaloglu Z, Oztosun M, et al. Effect of systemic steroid treatment on serum leptin levels in patients with COPD exacerbation. European Respiratory Journal 2005;26(Suppl 49):284s.
-
- Kunter E, Ilvan A, Ozmen N, Demirer E, Ozturk A, Avsar K, et al. Effect of corticosteroids on hemostasis and pulmonary arterial pressure during chronic obstructive pulmonary disease exacerbation. Respiration 2006;75(2):145‐54. - PubMed
-
- Kunter E, Ilvan A, Ozturk A, Demirer E, Ozmen N, Avsar K, et al. Effects of steroid treatment on hemodynamic and haemostatic parameters in COPD exacerbation. European Respiratory Journal 2005;26(Suppl 49):298s.
Li 2003 {published data only}
-
- Li H, He G, Chu H, Zhao L, Yu H. A step‐wise application of methylprednisolone versus dexamethasone in the treatment of acute exacerbations of COPD. Respirology 2003;8(2):199‐204. - PubMed
Mirici 2002 {published data only}
-
- Mirici A, Meral M, Akgun M, Tutar U, Kiris S. Comparison of the effectiveness of parenteral and nebuliser steroid in COPD exacerbations. European Respiratory Journal 2002;20(Suppl 38):243s.
Murata 1990 {published data only}
-
- Murata GH, Gorby MS, Chick TW, Halperin AK. Intravenous and oral corticosteroids for the prevention of relapse after treatment of decompensated COPD. Effect on patients with a history of multiple relapses. Chest 1990;98:845‐94. - PubMed
Plant 1999 {unpublished data only}
-
- Plant PK, Watson JP, Muers MF, Pearson SB. Randomised controlled trial of oral corticosteroids in acute exacerbations of COPD. Proceedings of the European Respiratory Society Annual Congress; 1999 Oct 9‐13; Madrid.
Rahman 2004 {published data only}
-
- Rahman M, Rahman M, Mamun S, Abdullah A, Haque A. Role of 7‐day and 14‐day courses of oral prednisolone treatment in acute exacerbation of COPD. Chest 2004;126(Suppl 4):839s.
Rizzato 1998 {published data only}
-
- Rizzato G. Effects of deflazacort hemisuccinate vs methylprednisolone succinate in COPD exacerbations. L'internista 1998;6:35‐40.
Roede 2008 {published data only}
-
- Roede Berendina Maria. Treatment of COPD Exacerbations in Primary and Secondary Care. [PhD thesis]. Amsterdam: University of Amsterdam, 2008:Chapter 3, 31‐47.
Sayiner 2001 {published data only}
-
- Sayiner A, Aytemur ZA, Cirit M, Unsal I. Systemic glucocorticoids in severe exacerbations of COPD. Chest 2001;119:726‐30. - PubMed
Shortall 2002 {published data only}
-
- Shortall SP, Blum J, Oldenburg FA, Rodgerson L, Branscombe JM, Harrow EM. Treatment of patients hospitalised for exacerbations of chronic obstructive pulmonary disease: comparison of an oral/metered‐dose inhaler regime and an intravenous/nebulizer regimen. Respiratory Care 2002;47(2):154‐8. - PubMed
Sin 2004 {published data only}
-
- Sin DD, Lacy P, Man SF. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2004;170(7):760‐5. - PubMed
Wang 2011 {published data only}
-
- Wang PH, Cheng SL, Wang HC, Chang HT, Hsu YL, Chen YS, et al. Systemic steroids in acute exacerbation of COPD ‐ from guidelines to bedside. International Journal of Clinical Pharmacology and Therapeutics 2011;49(11):705‐8. - PubMed
Additional references
Anthonisen 1987
-
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Annals of Internal Medicine 1987;106:196‐204. - PubMed
Bathoorn 2008
Buist 2007
-
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (The BOLD Study): a population‐based prevalence study. Lancet 2007;370(9589):741‐50. - PubMed
Chapman 2001
-
- Chapman KR, Tashkin DP, Pye DJ. Gender bias in the diagnosis of COPD. Chest 2001;119(6):1691‐5. - PubMed
Crockett 2001
-
- Crockett AJ, Cranston JM, Moss JR. Chronic Obstructive Pulmonary Disease (COPD) ‐ Economic Case Statement. Milton QLD: Lung Foundation Australia, 2001.
Donaldson 2002
Donaldson 2005
-
- Donaldson GC, Wilkinson TMA, Hurst JR, Perera WR, Wedzicha JA. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2005;171:446‐52. - PubMed
Donohue 2005
-
- Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005;2(1):111‐24. - PubMed
Effing 2009
-
- Effing TW, Kerstjens HAM, Monninkhof EM, Valk PDLPM, Wouters EFM, Postma DS, et al. Definitions of exacerbations: does it really matter in clinical trials on COPD?. Chest 2009;136(3):918‐23. - PubMed
Falk 2008
GOLD 2013
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/guidelines‐global‐strategy‐for‐diagnosis‐managem... (accessed 10 August 2014).
Groenewegen 2001
-
- Groenewegen K, Schols A, Wouters EFM. Prognosis after hospitalization for acute exacerbations of COPD. European Respiratory Journal 2001;8(Suppl 33):209s.
Henzen 2000
-
- Henzen C, Suter A, Lerch E, Urbinelli R, Schorno XH, Briner VA. Suppression and recovery of adrenal response after short‐term, high‐dose glucocorticoid treatment. Lancet 2000;355:542‐45. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hurst 2010
-
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal‐Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. New England Journal of Medicine 2010;363(12):1128‐38. - PubMed
Jeppesen 2012
Leidy 2010
-
- Leidy NK, Wilcox TK, Jones PW, Murray L, Winnette R, Howard K, et al. Development of the EXAcerbations of Chronic Obstructive Pulmonary Disease Tool (EXACT): a patient‐reported outcome (PRO) measure. Value in Health 2010;13(8):965‐75. - PubMed
Lindenauer 2010
-
- Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbations of chronic obstructive pulmonary disease. JAMA 2010;303:2359‐67. - PubMed
Lopez 2006
-
- Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. European Respiratory Journal 2006;27(2):397‐412. - PubMed
Mahler 1984
-
- Mahler D, Weinberg D, Wells C, Feinstein A. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 1984;85:751‐8. - PubMed
McEvoy 1997
-
- McEvoy CE, Niewoehner DE. Adverse effects of corticosteroid therapy for COPD. Chest 1997;111:732‐43. - PubMed
Murray 1997
-
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990‐2020: Global Burden of Disease Study. Lancet 1997;349(9064):1498‐504. - PubMed
NICE 2010
-
- National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care (partial update), 2010. www.guidance.nice.org.uk/cg101 (accessed 10 August 2014).
Oostenbrink 2004
-
- Oostenbrink JB, Rutten‐van Molken MP. Resource use and risk factors in high‐cost exacerbations of COPD. Respiratory Medicine 2004;98:883‐91. - PubMed
Papi 2006
-
- Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. American Journal of Respiratory and Critical Care Medicine 2006;173(10):1114‐21. - PubMed
Perret 2013
-
- Perret JL, Dharmage SC, Matheson MC, Johns DP, Gurrin LC, Burgess JA, et al. The interplay between the effects of lifetime asthma, smoking, and atopy on fixed airflow obstruction in middle age. American Journal of Respiratory and Critical Care Medicine 2013;187(1):42‐8. - PubMed
Ries 2005
-
- Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg Scale, and visual analog scale. COPD 2005;2(1):105‐10. - PubMed
Rodriguez‐Roisin 2000
-
- Rodriguez‐Roisin R. Toward a consensus definition for COPD exacerbations. Chest 2000;117:398s‐401. - PubMed
Schermer 2002
-
- Schermer T, Chavannes N, Saris C, Akkermans R, Schayck C, Weel C. Exacerbations and associated health care cost in patients with chronic obstructive pulmonary disease in general practice. Results from the COOPT trial. European Respiratory Journal 2002;20(Suppl 38):398s.
Seemungal 1998
-
- Seemungal TAR, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1998;157:1418‐22. - PubMed
Seemungal 2000
-
- Seemungal T, Donaldson G, Bhowmik A, Jefries D, Wedizicha J. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;161(5):1608‐13. - PubMed
Sethi 2008
-
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. New England Journal of Medicine 2008;359(22):2355‐65. - PubMed
Sherk 2000
Soler‐Cataluna 2005
Suissa 2012
Sullivan 2000
-
- Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest 2000;117:5s‐9s. - PubMed
Vestergaard 2007
-
- Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk in patients with chronic lung diseases treated with bronchodilator drugs and inhaled and oral corticosteroids. Chest 2007;132(5):1599‐607. [PUBMED: 17890464 ] - PubMed
Walters 2011
Wedzicha 2000
Wilkinson 2004
-
- Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2004;169(12):1298‐303. - PubMed
Wise 2005
-
- Wise RA, Brown CD. Minimal clinically important differences in the six‐minute walk test and the incremental shuttle walking test. COPD 2005;2(1):125‐9. - PubMed
References to other published versions of this review
Walters 2009
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

