The Top 10 oomycete pathogens in molecular plant pathology
- PMID: 25178392
- PMCID: PMC6638381
- DOI: 10.1111/mpp.12190
The Top 10 oomycete pathogens in molecular plant pathology
Abstract
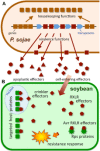
Oomycetes form a deep lineage of eukaryotic organisms that includes a large number of plant pathogens which threaten natural and managed ecosystems. We undertook a survey to query the community for their ranking of plant-pathogenic oomycete species based on scientific and economic importance. In total, we received 263 votes from 62 scientists in 15 countries for a total of 33 species. The Top 10 species and their ranking are: (1) Phytophthora infestans; (2, tied) Hyaloperonospora arabidopsidis; (2, tied) Phytophthora ramorum; (4) Phytophthora sojae; (5) Phytophthora capsici; (6) Plasmopara viticola; (7) Phytophthora cinnamomi; (8, tied) Phytophthora parasitica; (8, tied) Pythium ultimum; and (10) Albugo candida. This article provides an introduction to these 10 taxa and a snapshot of current research. We hope that the list will serve as a benchmark for future trends in oomycete research.
Keywords: diversity; genomics; microbiology; oomycetes plant pathology.
© 2014 BSPP AND JOHN WILEY & SONS LTD.
Figures











References
-
- Adhikari, T.B. , Liu, J.Q. , Mathur, S. , Wu, C.R.X. and Rimmer, S.R. (2003) Genetic and molecular analyses in crosses of race 2 and race 7 of Albugo candida . Phytopathology, 93, 959–965. - PubMed
-
- Agrios, G.N. (2005) Plant Pathology. Burlington, Massachusetts: Elsevier Academic Press Publications.
-
- Ah Fong, A.M.V. and Judelson, H.S. (2003) Cell cycle regulator Cdc14 is expressed during sporulation but not hyphal growth in the fungus‐like oomycete Phytophthora infestans . Mol. Microbiol. 50, 487–494. - PubMed
-
- Akinsanmi, O.A. and Drenth, A. (2013) Phosphite and metalaxyl rejuvenate macadamia trees in decline caused by Phytophthora cinnamomi . Crop Protect. 53, 29–36.
Publication types
MeSH terms
Grants and funding
- BB/M017834/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/M003809/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/K009176/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/H019820/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/E007120/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

