Protein Oxidation in Aging: Does It Play a Role in Aging Progression?
- PMID: 25178482
- PMCID: PMC4507125
- DOI: 10.1089/ars.2014.6062
Protein Oxidation in Aging: Does It Play a Role in Aging Progression?
Abstract
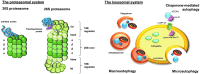
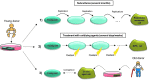
Significance: A constant accumulation of oxidized proteins takes place during aging. Oxidation of proteins leads to a partial unfolding and, therefore, to aggregation. Protein aggregates impair the activity of cellular proteolytic systems (proteasomes, lysosomes), resulting in further accumulation of oxidized proteins. In addition, the accumulation of highly crosslinked protein aggregates leads to further oxidant formation, damage to macromolecules, and, finally, to apoptotic cell death. Furthermore, protein oxidation seems to play a role in the development of various age-related diseases, for example, neurodegenerative diseases.
Recent advances: The highly oxidized lipofuscin accumulates during aging. Lipofuscin formation might cause impaired lysosomal and proteasomal degradation, metal ion accumulation, increased reactive oxygen species formation, and apoptosis.
Critical issues: It is still unclear to which extent protein oxidation is involved in the progression of aging and in the development of some age-related diseases.
Future directions: An extensive knowledge of the effects of protein oxidation on the aging process and its contribution to the development of age-related diseases could enable further strategies to reduce age-related impairments. Strategies aimed at lowering aggregate formation might be a straightforward intervention to reduce age-related malfunctions of organs.
Figures




References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, and Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25: 239–252, 2000 - PubMed
-
- Aeschbach R, Amado R, and Neukom H. Formation of dityrosine cross-links in proteins by oxidation of tyrosine residues. Biochim Biophys Acta 439: 292–301, 1976 - PubMed
-
- Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, and Markesbery WR. Protein oxidation in the brain in Alzheimer's disease. Neuroscience 103: 373–383, 2001 - PubMed
-
- Alberti S, Demand J, Esser C, Emmerich N, Schild H, and Hohfeld J. Ubiquitylation of BAG-1 suggests a novel regulatory mechanism during the sorting of chaperone substrates to the proteasome. J Biol Chem 277: 45920–45927, 2002 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

