Effects of the novel, selective and low-efficacy mu opioid receptor ligand NAQ on intracranial self-stimulation in rats
- PMID: 25178814
- PMCID: PMC4310756
- DOI: 10.1007/s00213-014-3719-7
Effects of the novel, selective and low-efficacy mu opioid receptor ligand NAQ on intracranial self-stimulation in rats
Abstract
Rationale: Low-efficacy mu opioid receptor agonists may be useful for some clinical indications, but clinically available low-efficacy mu agonists also have low selectivity for mu vs. kappa opioid receptors. NAQ (17-cyclopropylmethyl-3,14ß-dihydroxy-4,5α-epoxy-6α-[(3'-isoquinolyl)acetamido]morphinan) is a novel opioid receptor ligand with low-efficacy at mu receptors and greater mu-receptor selectivity than existing low-efficacy agonists.
Objectives: This study examined behavioral effects of NAQ in rats using an intracranial self-stimulation (ICSS) procedure that has been used previously to examine other opioids. NAQ effects were examined before, during, and after chronic morphine treatment, and effects of NAQ were compared to effects of nalbuphine and naltrexone.
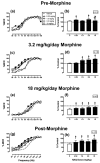
Methods: Adult male Sprague-Dawley rats were trained to respond for electrical brain stimulation delivered via electrodes implanted in the medial forebrain bundle. A range of brain stimulation frequencies maintained a wide range of baseline ICSS rates. Effects of NAQ (0.32-10 mg/kg), nalbuphine (1.0 mg/kg), and naltrexone (0.1 mg/kg) were determined before morphine treatment and during treatment with 3.2 and 18 mg/kg/day morphine. NAQ effects were also redetermined beginning 2 weeks after termination of morphine treatment.
Results: NAQ produced weak ICSS facilitation in morphine-naïve rats but more robust ICSS facilitation during and after morphine treatment and also reversed morphine withdrawal-associated depression of ICSS. These effects were similar to effects of nalbuphine.
Conclusions: These results agree with the in vitro characterization of NAQ as a low-efficacy mu agonist. Opioid exposure may enhance abuse-related effects of NAQ, but NAQ may also serve as a low-efficacy and relatively safe option for treatment of opioid withdrawal or dependence.
Conflict of interest statement
Disclosure: The authors declare no conflict of interest.
Figures
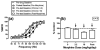


References
-
- Balster RL, Lukas SE. Review of self-administration. Drug Alcohol Depend. 1985;14:249–61. - PubMed
-
- Beguin C, Potter DN, Dinieri JA, Munro TA, Richards MR, Paine TA, Berry L, Zhao Z, Roth BL, Xu W, Liu-Chen LY, Carlezon WA, Jr, Cohen BM. N-methylacetamide analog of salvinorin A: a highly potent and selective kappa-opioid receptor agonist with oral efficacy. J Pharmacol Exp Ther. 2008;324:188–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

