Role of white adipose lipolysis in the development of NASH induced by methionine- and choline-deficient diet
- PMID: 25178843
- PMCID: PMC4188754
- DOI: 10.1016/j.bbalip.2014.08.015
Role of white adipose lipolysis in the development of NASH induced by methionine- and choline-deficient diet
Abstract
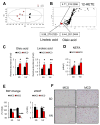
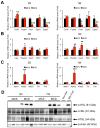
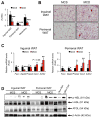
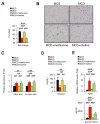
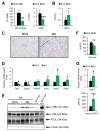
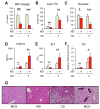
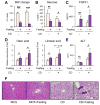
Methionine- and choline-deficient diet (MCD) is a model for nonalcoholic steatohepatitis (NASH) in rodents. However, the mechanism of NASH development by dietary methionine/choline deficiency remains undetermined. To elucidate the early metabolic changes associated with MCD-NASH, serum metabolomic analysis was performed using mice treated with MCD and control diet for 3 days and 1 week, revealing significant increases in oleic and linoleic acids after MCD treatment. These increases were correlated with reduced body weight and white adipose tissue (WAT) mass, increased phosphorylation of hormone-sensitive lipase, and up-regulation of genes encoding carboxylesterase 3 and β2-adrenergic receptor in WAT, indicating accelerated lipolysis in adipocytes. The changes in serum fatty acids and WAT by MCD treatment were reversed by methionine supplementation, and similar alterations were detected in mice fed a methionine-deficient diet (MD), thus demonstrating that dietary methionine deficiency enhances lipolysis in WAT. MD treatment decreased glucose and increased fibroblast growth factor 21 in serum, thus exhibiting a similar metabolic phenotype as the fasting response. Comparison between MCD and choline-deficient diet (CD) treatments suggested that the addition of MD-induced metabolic alterations, such as WAT lipolysis, to CD-induced hepatic steatosis promotes liver injury. Collectively, these results demonstrate an important role for dietary methionine deficiency and WAT lipolysis in the development of MCD-NASH.
Keywords: Choline deficiency; Fasting response; Linoleic acid; Lipolysis; Metabolomics; Oleic acid.
Published by Elsevier B.V.
Conflict of interest statement
Figures

References
-
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. - PubMed
-
- Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. - PubMed
-
- Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. - PubMed
-
- Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

